* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 5 (part 4) Enzyme Regulation
Lipid signaling wikipedia , lookup
Biochemical cascade wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Epitranscriptome wikipedia , lookup
Proteolysis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Restriction enzyme wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Metalloprotein wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Biochemistry wikipedia , lookup
Phosphorylation wikipedia , lookup
Citric acid cycle wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Catalytic triad wikipedia , lookup
Biosynthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
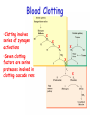
Chapter 5 (part 4) Enzyme Regulation Regulation of Enzyme Activity Enzyme quantity – regulation of gene expression (Response time = minutes to hours) a) Transcription b) Translation c) Enzyme turnover Enzyme activity (rapid response time = fraction of seconds) a) Allosteric regulation b) Covalent modification c) Association-disassociation’ d) Proteolytic cleavage of proenzyme Allosteric Regulation • End products are often inhibitors • Allosteric modulators bind to site other than the active site • Allosteric enzymes usually have 4o structure • Vo vs [S] plots give sigmoidal curve for at least one substrate • Can remove allosteric site without effecting enzymatic action Regulation of Enzyme Activity (biochemical regulation) • 1st committed step of a biosynthetic pathway or enzymes at pathway branch points often regulated by feedback inhibition. 1 A 2 C B X H 4” I 3” 3’X 4’ E F 5” 5’ J G • Efficient use of biosynthetic precursors and energy Phosphofructokinase( PFK) Fructose-6-P + ATP -----> Fructose-1,6-bisphosphate + ADP •PFK catalyzes 1st committed step in glycolysis (10 steps total) (Glucose + 2ADP + 2 NAD+ + 2Pi 2pyruvate + 2ATP + 2NADH) •Phosphoenolpyruvate is an allosteric inhibitor of PFK •ADP is an allosteric activator of PFK Allosteric modulators bind to site other than the active site and allosteric enzymes have 4o structure Fructose-6-P + ATP -----> Fructose-1,6-bisphosphate + ADP ADP Allosteric Activator (ADP) binds distal to active site Vo vs [S] plots give sigmoidal curve for at least one substrate Binding of this allosteric inhibitor or this activator does not effect Vmax, but does alter Km Allosteric enzyme do not follow M-M kinetics Allosteric T to R transition ET-I I I ET Concerted model ER S Sequential model S ER-S Covalent modification •Regulation by covalent modification is slower than allosteric regulation •Reversible •Require one enzyme for activation and one enzyme for inactivation •Covalent modification freezes enzyme T or R conformation Phosphorylation /dephosphorylation •most common covalent modification • involve protein kinases/phosphatase •PDK inactivated by phosphorylation •Amino acids with –OH groups are targets for phosphorylation •Phosphates are bulky (-) charged groups which effect conformation Enzyme Regulation by Association/Disassociation •Acetyl-CoA Carboxylase •acetyl-CoA + CO2 + ATP malonyl-CoA + ADP + Pi •1St committed step in fatty acid biosynthesis •In presence of citrate activated •In presence of fatty acyl-CoA inactivated citrate unpolymerized Fatty acyl-CoA polymerized Proteolytic cleavage of proenzyme(zymogen) Proinsulin to Insulin Blood Clotting •Clotting involves series of zymogen activations •Seven clotting factors are serine proteases involved in clotting cascade rxns X X X X X X