* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Searching for Binding Partners for the Novel PHKG1 Variant, PhKγ
Deoxyribozyme wikipedia , lookup
Paracrine signalling wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Real-time polymerase chain reaction wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Community fingerprinting wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Proteolysis wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Molecular cloning wikipedia , lookup
Signal transduction wikipedia , lookup
Expression vector wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Phosphorylation wikipedia , lookup
Restriction enzyme wikipedia , lookup
Biochemistry wikipedia , lookup
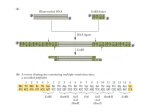
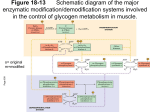
SEARCHING FOR BINDING PARTNERS FOR THE NOVEL PHKG1 VARIANT, PhK-γ181 KISHORE POLIREDDY, Western Kentucky University. Kinases? What do they do? Kinases are enzymes which transfer a phosphate group from ATP to a specific substrate, this process is known as Phosphorylation. PO3-2 kinases ATP ADP Structure of Phosphorylase kinase Alpha,β regulatory subunits Gamma is catalytic subunit Delta subunit is intrinsic molecule calmodulin Importance of glycogen Glycogen is the major carbohydrate reserve, composed of linked glucose molecules stored within the liver and muscle. PhK is the rate limiting enzyme of the glycogenolysis. Role of PhK in glycogen breakdown Alternate processing of PHKG1 PHKG1-mRNA codes for 387 amino acids gamma subunit Alternate processing of PHKG1-mRNA will code for 181 amino acid gamma subunit. Calmodulin-binding (domain-N) catalytic domain-PHKG1 PHKG1 PHKG1-human 387 aa Calmodulin-binding (domain-C). Truncated form, PhKγ-181, retains the kinase activity and its activity is influenced by the Phosphorylation of PKC. Aims of the present study To identify the potential binding partners for γ-181 by using Lex-A based yeast two hybrid system. Current Study Presently, we are trying to produce a LexA- γ181 fusion protein by using the vector pLex-A (bait) and the vector pB42 (prey) to search for binding partners within a brain cDNA library. Interaction between γ-181 and the brain cDNA library proteins will be monitored using Lac-Z and LEU2 as reporter genes. Future Work Cloning of γ-181 in to pLex-A vector Identify the clones which shows positive interaction between γ-181, c-DNA library proteins Library plasmids isolated from the clones that show positive results on the yeast two hybrid screen will be sequenced to identify novel binding partners for γ-181. Preliminary results PCR amplification of γ-181 1 2 3 1. Marker 2. PCR amplified product1 3. PCR amplified product 2 Restriction digestion of vector 11 2 3 4 5 1. Marker 2. Uncut pLexA vector 3. Restriction digestion pLexA vector with EcoR1. 4. Restriction digestion pLexA vector with BamH1 5. Restriction digestion pLexA vector with EcoR1 & BamH1 Ligation of restriction digested γ-181 in to predigested pLex-A vector. Restriction digested γ-181 Restriction digested vector Ligation γ-181 pLex-A vector Acknowledgements • Dr. Nancy A Rice • Dr. B. Sharma KBRIN References 1. Three-Dimensional Structure of Phosphorylase Kinase at 22A Resolution and Its Complex with Glycogen Phosphorylase b. Structure, Vol. 10, 33–41, January, 2002, Elsevier Science Ltd. 2. The Fractal Structure of Glycogen: A Clever Solution to Optimize Cell Metabolism. Biophysical Journal Volume 77 September 1999 1327–1332. 3. The crystal structure of a phosphorylase kinase peptide substrate complex: kinase substrate recognition. The EMBO Journal Vol.16 No.22 pp.6646–6658, 1997. 4. A Eukaryotic Transcriptional Activator Bearing the DNA Specificity of a Prokaryotic Repressor. Cell, Vol. 43, 729–736, December, 1985. Thank you