* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Expression and Purification of Recombinant Protein in bacteria and
Cancer epigenetics wikipedia , lookup
Microevolution wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Epigenomics wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Designer baby wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Extrachromosomal DNA wikipedia , lookup
Point mutation wikipedia , lookup
History of genetic engineering wikipedia , lookup
Protein moonlighting wikipedia , lookup
Molecular cloning wikipedia , lookup
Helitron (biology) wikipedia , lookup
DNA vaccination wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
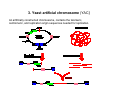
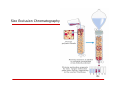
Expression and Purification of Recombinant Protein in bacteria and Yeast Presented By: Puspa pandey, Mohit sachdeva & Ming yu DNA Vectors Molecular carriers which carry fragments of DNA into host cell. Usually derived from plasmids, which are small, circular, doublestranded DNA molecules Some widely used vectors: 1. 2. 3. 4. 5. 6. 7. Plasmids Cosmids Yeast Artificial Chromosomes Phage lambda vectors Bacterial Artificial Chromosomes Human Artificial Chromosomes Transposons etc. 1. Plasmids Extrachromosomal DNA mainly found in bacteria, capable of autonomous replication Typically circular and double-stranded Advantages as a Vector They are derived from natural plasmids that occur in bacterial cells so easier to manipulate and propagate than other vectors. More stable vector to maintain a particular clone Great variation in size( from 1 to over 400 kb) Disadvantages Small fragments (10-15 kb) of DNA can be inserted. Low transformation efficiency 2. Cosmids Artificially constructed cloning vectors containing the cos gene of the phage lambda. Can be packaged into lambda phage particles for infection into Bacteria. Advantages It can carry Larger fragments (40-50 kb) of DNA High transformation efficiency Usually used in making the Genomic libraries. Disadvantages Unable to accept more than 40-50 kbp of DNA Needs sophisticated process for preparation 3. Yeast artificial chromosome (YAC) An artificially constructed chromosome, contains the telomeric, centromeric, and replication origin sequences needed for replication. Advantages Capable of carrying a large DNA fragment (up to 2 Mb) One can get directly the eukaryotic protein products Disadvantages Transformation efficiency is very low Not possible to recover large amount of pure insert DNA from individual clone. Unstable sometimes, producing chimeric effects Making and Purification of Recombinant Protein in bacteria and Yeast Recombinant Protein A protein obtained by introducing recombinant DNA into a host (microorganism or yeast cell) and causing it to produce the gene product. OR A protein whose amino acid sequence is encoded by a cloned gene. Basic steps to get recombinant Protein: 1. Amplification of gene of interest. ( Using PCR). 2. Insert into cloning vector. (Ex: PCR*8). 3. Sub cloning into expression vector. (Ex: pKK223-3 or PSVK 3) 4. Transformation into protein expressing bacteria (E coli) or yeast. 5. Test for identification of recombinant protein.( Western blot or Fluoroscence) 6. Large scale production. (Large scale fermentor) 7. Isolation and purification. Various factors to be considered: 1. Which expression vector ? 2. Which host system? 3. Properties of protein • Membrane bound • Solubility • Single or multidomain • Size? 4. Where it expressed? 5. How to identify, isolate and purification method? Necessary Features of expression vector: •Origin of replication •Drug resistance marker •A promoter •Transcription terminator •Restriction sites Expression Vectors with or without specific tags…… Vectors for non-fusion proteins Vectors for fusion proteins pKK223-3 pSVK 3 PSVL SV40 pGEX pQE pET pEZZ 18 Features of small tags (Fusion proteins): 1.Improved expression ex: sumo fusions 2.Improved solubility 3.Cell compartments can be targeted 4.Improved detection ex: GFP tag fluoroscence, specific antibodies detection in western 5.Improved purification ex: using ionexchange or affinity chromatography For small peptides Large peptides Poly his Flag Myc Maltose binding protein( MBP) Chitin binding domain Cellulose binding domain Glutathione S- transferase( GST) Features of Host --prokaryotic systems ex: E coli Advantages •Many references and much experience available e.g. E. coli. •Wide choice of cloning vectors. •Gene expression easily controlled. •Easy to grow with high yields. •Product can be designed for secretion into the growth media. Disadvantages •No post-translational modification. •Biological activity and immunogenicity may differ from natural protein. •High endotoxin content in gram negative. Features of eukaryotic systems: yeast Advantages •Lacks detectable endotoxins. •Fermentation relatively inexpensive. •Facilitates glycosylation and formation of disulphide bonds. •Only 0.5% native proteins are secreted so isolation of secreted product is simplified. •Well established large scale production and downstream processing. Disadvantage •Gene expression less easily controlled. •Glycosylation not identical to mammalian systems. Isolation of protein: Initial steps prior to purification: Disruption of cells: Osmotic Chemical Enzymatic Mechanical Clarification: Centrifugation Filtration Concentration: Precipitation Ultra filtration Differential centrifugation Differential centrifugation: It’s a procedure in which a homogenate is subjected to repeated centrifugations each time increasing the centrifugal force. Separation is based predominantly on particle mass and size with larger and heavier particles pelleting at lower centrifugal fields For specific organelle sub cellular fractions. Purification of isolated proteins Various purification methods: 1. Charge: IEC/IEF 2. Size: size exclusion chromatography 3. Hydrophobicity: Hydrophobic Interaction Chromatography 4. Ligand specifity: affinity chromatography, nucleotide and coenzymes resins, phosphoprotein resins. 5. Avidin biotin matrices 6. Carbohydrate binding 7. Dye resins. 8. Solubility: Precipitation Precipitation Proteins tend to precipitate at their isoelectric point if the ionic strength of the solution is very high; First step in protein purification; Ammonium sulfate and Trichloroacetate (TCA) are the most common salt. Buffer Exchange Importance: Different purification techniques require the protein present in buffers of specific pH and ionic strengths. Size Exclusion Chromatography Ion Exchange Chromatography Affinity Chromatography Hydrophobic Interaction Chromatography (HIC) It is based on hydrophobic attraction between the stationary phase and the protein molecules. The stationary phase consists of small non-polar groups ( butyl, octyl or phenyl) attached to a hydrophilic polymer backbone (cross-linked dextran or agarose, for example). The sample is loaded in a buffer containing a high concentration of a non-denaturing salt (frequently ammonium sulfate). The proteins are then eluted as the concentration of the salt in the buffer is decreased. Purification of Tagged Recombinant Proteins Ni-NTA Agarose To purify recombinant protein containing polyhistidine (6xHis) sequence. Streptavidin Agarose To purify biotinylated recombinant protein . Thanks!