* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 22 - Introduction to Metabolism: Regulation Key Concepts
Two-hybrid screening wikipedia , lookup
Secreted frizzled-related protein 1 wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Gene regulatory network wikipedia , lookup
Citric acid cycle wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Proteolysis wikipedia , lookup
Biosynthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Lipid signaling wikipedia , lookup
Signal transduction wikipedia , lookup
Biochemistry wikipedia , lookup
Paracrine signalling wikipedia , lookup
Metabolic network modelling wikipedia , lookup
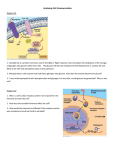
Bioc 460 - Dr. Miesfeld Fall 2008 Lecture 22 - Introduction to Metabolism: Regulation The DC metro system Key Concepts • Six major groups of metabolic pathways • Regulation of metabolic flux • Glucagon, epinephrine, and insulin signaling pathways KEY CONCEPT QUESTIONS: What mechanisms control flux through metabolic pathways? How do glucagon, epinephrine, and insulin control glucose levels? Overview of metabolic pathways Small biomolecules serve as metabolites (reactants and products) in biochemical reactions within cells that are required for life-sustaining processes. Enzymes (either protein or RNA) are the chemical catalysts Figure 1. that drive these biochemical reactions. In these enzymemediated biochemical reactions, the products of one reaction are inevitably the reactants for other functionallyrelated reactions. The thousands of reactions in a cell required for sustaining life are interdependent and highly regulated to maximize efficient use of limiting metabolic resources. The emerging discipline of systems biology attempts to describe complex chemical reaction networks in cells using mathematical models that are able to predict metabolic flux (reactant and product concentrations over time) in response to environmental or physiological conditions. Systems biology can provide a better paradigm for presenting a global picture of cellular metabolism, it is more instructive to focus on a limited number of biochemical reactions that have been highly characterized and which provide a basis for understanding the chemistry of life. Using this approach, sets of Figure 2. biochemical reactions are grouped together into metabolic pathways (figure 1 ). In order to understand how a metabolic pathway is organized, let's first look at a mini-pathway consisting of just two reactions. As shown in figure 2, the reactants aspartate and citrulline are metabolites in the urea cycle which is a metabolic pathway in liver cells responsible for nitrogen excretion in animals. The enzyme argininosuccinate synthase is a protein that catalyzes a condensation reaction that forms the product argininosuccinate using phosphoryl transfer energy made available by ATP. In the second reaction, argininosuccinate is cleaved by the enzyme argininosuccinate lyase to form the products fumarate and arginine. The chemical difference between citrulline and asparagine is the addition of a single amino group obtained from aspartate, however in order for this to occur, argininosuccinate has to function both as a product and a reactant. 1 of 8 pages Bioc 460 - Dr. Miesfeld Fall 2008 An example of a more complex metabolic pathway is Figure 3. illustrated in figure 3 where it can be seen that enzymes interconvert metabolites using reversible and irreversible reactions. In some reactions, phosphoryl bond energy available in ATP is used to drive the reaction toward product formation. Three types of linked reactions are commonly found in metabolism. The most common type is linear pathways in which each reaction generates only a single product that is a reactant for the next reaction in the pathway. In contrast, forked pathways usually generate two products, each of which as a different metabolic fate. Lastly, cyclic pathways contain a number of metabolites that are regenerated during each turn of the cycle, serving as both reactants and products in every reaction. Two examples of cyclic pathways we will discuss later in the course are the citrate cycle and the urea cycle. Note that the flux of metabolites through metabolic pathways is dependent on both the activity of each Figure 4. enzyme in the pathway, and the intracellular concentrations of reactants and products which affects the directionality of reactions as a function of mass action (Le Chatelier's principle). Figure 4 shows the basic metabolic map we will use in this course which is designed to highlight the interdependence of six major groups of pathways (both anabolic and catabolic). A full page copy of this metabolic map can be downloaded from the course website. The layout of pathways in this version of a metabolic map was designed to illustrates the hierarchical nature of metabolism which includes four classes of macromolecules (proteins, nucleic acids, carbohydrates, and lipids), six primary metabolites (amino acids, nucleotides, fatty acids, glucose, pyruvate, acetyl CoA) and seven small biomolecules (NH4+, CO2, NADH, FADH2, O2, ATP, H2O), all of which we will encounter frequently on our journey. The metabolic chart in figure 4 will be used as a template each time a new pathway is introduced using the “divide and conquer” 2 of 8 pages Bioc 460 - Dr. Miesfeld Fall 2008 strategy illustrated in figure 5. We will start by describing Figure 5. pathways involved in energy conversion processes (glycolysis, citrate cycle, electron transport system, oxidative phosphorylation, photophosphorylation, and carbon fixation), and then survey both degradative and biosynthetic pathways in carbohydrate metabolism (pentose phosphate pathway, gluconeogenesis, glycogen degradation and synthesis), lipid metabolism (fatty acid oxidation and synthesis, lipoprotein functions, steroid and eicosanoid synthesis), and amino acid metabolism (amino acid degradation and synthesis). Figure 6 summarizes the road map we will use for these lectures. The boundaries we have drawn to separate the six major groups of pathways are somewhat arbitrary. Nevertheless, I have chosen this particular pedagogical strategy for two reasons, 1) it underscores the importance of energy conversion as the foundation for all other metabolic pathways; without sunlight and the fuel produced by photosynthetic organisms, life would not exist on this planet as we know it, and 2) it permits us to maintain our focus on the role of proteins in biochemical processes, in this case, enzyme regulation of metabolic flux through linked reactions in a pathway. We will start off the discussion of major pathways in metabolism by answering the following four questions: 1. What does the pathway accomplish for the cell? 2. What is the overall net reaction of the pathway? 3. What are the key enzymes in the pathway? 4. What are examples of this pathway in real life? Figure 6. The answers to these four simple questions provides you with the basic information you will need to study the ten individual pathways we will cover as shown below: Pathway(s) Glycolysis Citrate Cycle Oxidative Phosphorylation Photosynthesis Pentose Phosphate Pathway Gluconeogenesis Glycogen Degradation and Synthesis Fatty Acid Degradation and Synthesis Nitrogen fixation and assimilation Urea Cycle Lectures 23, 24 25, 26 27, 28 29, 30 32 32 33 34, 35 37 37 3 of 8 pages Bioc 460 - Dr. Miesfeld Fall 2008 Regulation of metabolic flux As described in lecture 21, catabolic pathways are defined as the collection of enzymatic reactions in the cell that lead to the degradation of macromolecules and nutrients for the purpose of energy capture, usually in the form of ATP and reducing power (NADH and FADH2). In contrast, anabolic pathways utilize energy available from the hydrolysis of ATP and the oxidation of reducing equivalents (primarily NADPH) to synthesize biomolecules for the cell. Importantly, catabolic and anabolic pathways are active at the same time in the cell and many metabolites serve as both substrates and products for different enzymes. Sometimes the catabolic pathways are more active than the opposing anabolic pathway, whereas at other times, anabolic pathways predominate when energy stores are plentiful and the necessary building blocks are available. The flow or flux of metabolites through catabolic and anabolic pathways is determined by two primary factors, 1) availability of substrates (diet or stored reserves) and 2) level of enzyme activity which is controlled by enzyme levels (gene transcription and protein synthesis), catalytic activity (allosteric control and covalent modification), and compartmentation (subcellular or tissue selective localization). As was described in the first half of the course, enzymes function by providing a suitable reaction environment (the enzyme active site) that serves to lower the energy of activation for a given reaction. Enzymes cannot change the equilibrium of a reaction, but instead, function as catalysts that increase reaction rates. With this in mind, reversible reactions in metabolism normally function near equilibrium and are therefore controlled by substrate availability, whereas, irreversible reactions are catalyzed by highly-regulated enzymes that function far from equilibrium. One of the best ways to understand how flux through various catabolic and anabolic pathways changes in response to substrate concentration and enzyme activity levels is to look at glucose metabolism in the liver before and after breakfast. The two primary hormones that control serum glucose levels are glucagon, the "I am hungry" hormone, and insulin, the "I just at ate" hormone. A third hormone that controls glucose levels in the blood is epinephrine (adrenaline), which I call the "I just saw a snake" hormone. Epinephrine signaling through β2 adrenergic receptors in liver cells also leads to the release of glucose by a similar mechanism. As illustrated in figure 7, in the morning before your first meal, blood glucose levels begin to decline after a night of “fasting” which triggers glucagon release from the pancreas. Glucagon signaling in liver cells activates both a catabolic pathway (glycogen degradation) and an anabolic pathway (gluconeogenesis), while at the same time inhibiting the catabolism of glucose by the glycolytic pathway. However, within an hour of eating a bowl of cereal and drinking a cup of fruit Figure 7. 4 of 8 pages Bioc 460 - Dr. Miesfeld Fall 2008 juice, your insulin levels increase due to elevated blood glucose causing activation of the insulin signaling pathway and stimulation of glucose uptake, glycogen synthesis, and an increase in glucose catabolism by the glycolytic pathway. Therefore, by “breaking your fast” you initiate a transient shift in flux through these various metabolic pathways until blood glucose levels stabilize around 5 mM. Importantly, the four pathways shown in figure 7 are active in liver cells all of the time, with the only change being the relative metabolite flux through each pathway in response to glucose concentrations and hormone activation of key enzymes. Figure 8. Glucagon, epinephrine, and insulin signaling Since glucagon, epinephrine, and insulin signaling will be used numerous times as examples of metabolic regulation during this half of the course, we need to take a closer look at how these two peptide hormones transmit intracellular signals. Glucagon signals through a G protein-coupled receptor pathway that activates protein kinase A (PKA) as a result of increased levels of the second messenger cyclic AMP. In contrast, insulin hormone signals through a receptor tyrosine kinase mechanism involving activation of phosphoinositol-3 kinase (PI-3K). Importantly, glucagon and Figure 9. epinephrine signaling (figure 8), and insulin signaling (figure 9), control glucose levels in the blood by regulating glycogen metabolism and gluconeogenesis. Note that insulin signaling also activates a separate pathway involving the small G protein Ras which controls cell proliferation. Glucagon is released by the pancreas and has been called the hunger hormone because it signals low blood glucose levels. Consistent with this physiological role, glucagon receptors are primarily expressed in liver and adipocyte cells where energy stores in the form of glycogen and fatty acid are located. Epinephrine-induced glycogen breakdown in muscle cells produces glucose as source of chemical energy to generate ATP which is needed for muscle contraction. Regulation of glycogen breakdown in liver cells by epinephrine and glucagon is dependent on the presence of β2 adrenergic and glucagon receptors, respectively. The biochemistry of these two signaling mechanisms is essentially identical with both receptor systems utilizing the same Gsα signaling pathway to stimulate adenylate cyclase (figure 8). Keep in mind that the physiological stimuli responsible for epinephrine secretion by the adrenal medulla (fight or flight response), and glucagon release from the pancreas (low blood glucose levels), are somewhat distinct and therefore these pathways are not usually stimulated in liver cells at the same time. 5 of 8 pages Bioc 460 - Dr. Miesfeld Fall 2008 As shown in figure 10, stimulation of adenylate cyclase activity by activated Gsα, leads to production of cAMP and subsequent Figure 10. dissociation of the PKA regulatory and catalytic subunits. As described in more detail in lecture 33, the two key regulatory enzymes in glycogen metabolism are phosphorylase kinase and glycogen synthase, both of which are phosphorylated in liver cells by PKA. Phosphorylation of phosphorylase kinase by PKA is an activating signal that transmits the epinephrine signal downstream through phosphorylation of its target protein glycogen phosphorylase. Glycogen phosphorylase catalyzes the removal of glucose units from glycogen through a phosphorolysis reaction (lecture 33). In contrast, PKA phosphorylation of glycogen synthase is an inhibitory signal that reduces enzyme activity and leads to a decrease in glycogen biosynthesis. The net result of PKA-mediated phosphorylation of phosphorylase kinase and glycogen synthase is increased glucose export to peripheral tissues. In summary, activation of the epinephrine or glucagon G protein-coupled receptors in liver cells results in increased intracellular levels of cAMP which initiates a PKA-dependent phosphorylation cascade culminating in glucose export. Importantly, the phosphorylation cascade involves both the activation and inhibition of downstream target proteins, that together, provide a rapid response to external stimuli (fear or hunger). The insulin receptor is synthesized as a single polypeptide chain that is processed by proteolytic cleavage to produce a disulfide-linked protein. The mature insulin receptor is an α2β2 heterotetramer consisting of cross-linked α and β chains connected by disulfide bridges. The α subunits form the extracellular ligand binding domain and the β subunits encode the intracellular protein tyrosine kinase function. Insulin binding to α subunits causes a conformational change in the receptor that stimulates the protein tyrosine kinase activity in the β subunits, resulting in 6 of 8 pages Bioc 460 - Dr. Miesfeld Fall 2008 receptor Figure 11. autophosphorylation. Activation of insulin receptor tyrosine kinase activity leads to tyrosine phosphorylation of a family of proteins called insulin receptor substrate (IRS) proteins that bind to the β subunits (figure 11). Recruitment of PI-3 kinase to the plasma membrane by IRS proteins stimulates phosphorylation of phosphatidylinositol 4,5bisphosphate (PIP2) to produce phosphatidylinositol 3,4,5-triphosphate (PIP3) which remains in the plasma membrane and serves as a docking site for signaling proteins containing pleckstrin homology (PH) domains. PH domains are highly conserved protein structural motifs that serve to recruit signaling proteins to the plasma membrane. Two proteins in the insulin signaling pathway containing PH domains are phosphoinositol-dependent kinase (PDK1), and Akt, a serine-threonine kinase originally identified as an oncogene gene (cancer gene) in the Akt8 murine retrovirus. Akt is also known as protein kinase B (PKB) because it shares amino acid homology with protein kinase A and protein kinase C. Both PDK1 and Akt bind to PIP3 leading to PDK1 phosphorylation of Akt and subsequent dissociation of activated Akt from PIP3. Akt diffuses through the cytosol where it phosphorylates numerous downstream targets. The net result is glucose uptake and increased glycogen synthesis to store the glucose for use later. Insulin signaling is similar in liver, muscle, and adipose tissue. Since glycogen phosphorylase and glycogen synthase have opposing effects on glycogen metabolism, it is critical that their activities be reciprocally regulated to avoid futile cycling and to efficiently control glucose-6P concentrations within the cell. Figure 12 summarizes the effects of glucagon and insulin signaling on glycogen metabolism in liver cells where it can be seen that glucagon stimulates glucose efflux and insulin stimulates glucose influx through the GLUT2 glucose transporter protein. It can be seen that net phosphorylation drives glycogen degradation and net dephosphorylation drives glycogen synthesis. 7 of 8 pages Bioc 460 - Dr. Miesfeld Fall 2008 ANSWERS TO KEY CONCEPT QUESTIONS: Figure 17. What mechanisms control flux through metabolic pathways? Substrate availability and enzyme activity levels control flux through metabolic pathways. Substrate availability is determined by both diet and release of stored metabolites, primarily glucose and fatty acids, although amino acids can also become available from increased rates of protein degradation. Enzyme activities are controlled by enzyme levels (gene expression protein synthesis, and rates of protein turnover), catalytic activity (allosteric control and covalent modification), and by compartmentation (subcellular organelles and tissue-selective localization). Changes in diet or physical activity affect the rate of metabolic flux in animals, whereas, environmental changes such as temperature, water, or sunlight, affect the rate of metabolic flux in plants and bacteria. The most highly regulated enzymes in metabolic pathways are those that control rate-limiting reactions, or catalyze the committed step in a pathway. The committed step in the pathway is one in which the product of the reaction has only one metabolic fate, and is thereby committed to be metabolized by the next enzyme in the pathway. How do glucagon, epinephrine, and insulin control glucose levels? Glucagon, epinephrine, and insulin are hormones that bind to membrane receptors on target cells and activate intracellular signaling pathways. Glucagon and epinephrine (adrenaline) signal the blood glucose levels are low (glucagon), or that glucose is needed quickly for muscle contraction (epinephrine). Both glucagon and epinephrine bind to G protein coupled receptors that activate second messenger-dependent pathways. Glucagon receptors stimulate Gsα proteins which activate adenylylate cyclase and produce high intracellular levels of cyclic AMP (cAMP). Epinephrine binding to β2 adrenergic receptors in liver cells also activates Gsα signaling, however, epinephrine binding to α1 adrenergic receptors in muscle cells activates phospholipase C signaling. cAMP activation of protein kinase A (PKA) activity leads to the phosphorylation of downstream target proteins, which together, lead to the accumulation of glucose through activation of both glycogen degradation and gluconeogenesis. Increased insulin levels signals to cells that glucose is plentiful and it should be metabolized to generate ATP and also stored for later use by building up glycogen levels. Insulin binds to a receptor tyrosine kinase on target cells and initiates a signaling cascade in involving the enzyme phosphoinositol-3 kinase (PI-3K) which phosphorylates another kinase called AKT which controls glucose metabolism through several mechanisms. Insulin activates glucose important and glycogen synthesis. 8 of 8 pages