* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Nucleic Acid metabolism De Novo Synthesis of Purine
Enzyme inhibitor wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Catalytic triad wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Genetic code wikipedia , lookup
Microbial metabolism wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Butyric acid wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Oligonucleotide synthesis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Biochemistry wikipedia , lookup
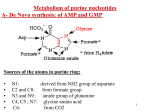
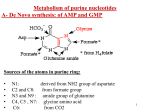
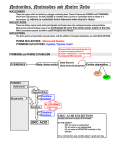
Nucleic Acid metabolism • De Novo Synthesis of Purine Nucleotides • We use for purine nucleotides the entire glycine molecule (atoms 4, 5,7), the amino nitrogen of aspartate (atom 1), amide nitrogen of glutamine (atoms 3, 9), components of the folate-one-carbon pool(atoms 2, 8), carbon dioxide, ribose 5-P from glucose and a great deal of energy in the form of ATP. In de novo synthesis, IMP is the first nucleotide formed. It is then converted to either AMP or GMP. • PRPP • Since the purines are synthesized as the ribonucleotides, (not as the free bases) a necessary prerequisite is the synthesis of the activated form of ribose 5-phosphate. Ribose 5-phosphate reacts with ATP to form 5Phosphoribosyl-1-pyrophosphate (PRPP). • This reaction occurs in many tissues because PRPP has a number of roles - purine and pyrimidine nucleotide synthesis, salvage pathways, NAD and NADP formation. The enzyme is heavily controlled by a variety of compounds (di- and tri-phosphates, 2,3-DPG), presumably to try to match the synthesis of PRPP to a need for the products in which it ultimately appears • Commitment Step • De novo purine nucleotide synthesis occurs actively in the cytosol of the liver where all of the necessary enzymes are present as a macromolecular aggregate. The first step is a replacement of the pyrophosphate of PRPP by the amide group of glutamine. The product of this reaction is 5-Phosphoribosylamine. The amine group that has been placed on carbon 1 of the sugar becomes nitrogen 9 of the ultimate purine ring. This is the commitment and ratelimiting step of the pathway • Control of De Novo Synthesis • Control of purine nucleotide synthesis has two phases. Control of the synthesis as a whole occurs at the amidotransferase step by nucleotide inhibition and/or [PRPP]. The second phase of control is involved with maintaining an appropriate balance (not equality) between ATP and GTP. Each one stimulates the synthesis of the other by providing the energy. Feedback inhibition also controls the branched portion as GMP inhibits the conversion of IMP to XMP and AMP inhibits the conversion of IMP to adenylosuccinate. • De Novo Synthesis of Pyrimidine Nucleotides • Since pyrimidine molecules are simpler than purines, so is their synthesis simpler but is still from readily available components. Glutamine's amide nitrogen and carbon dioxide provide atoms 2 and 3 or the pyrimidine ring. They do so, however, after first being converted to carbamoyl phosphate. The other four atoms of the ring are supplied by aspartate. As is true with purine nucleotides, the sugar phosphate portion of the molecule is supplied by PRPP. • Carbamoyl Phosphate • Pyrimidine synthesis begins with carbamoyl phosphate synthesized in the cytosol of those tissues capable of making pyrimidines (highest in spleen, thymus, GItract and testes). This uses a different enzyme than the one involved in urea synthesis. Carbamoyl phosphate synthetase II (CPS II) prefers glutamine to free ammonia and has no requirement for N-Acetylglutamate • Formation of Orotic Acid • Carbamoyl phosphate condenses with aspartate in the presence of aspartate transcarbamylase to yield N-carbamylaspartate which is then converted to dihydroorotate. • In man, CPSII, asp-transcarbamylase, and dihydroorotase activities are part of a multifunctional protein. • Oxidation of the ring by a complex, poorly understood enzyme produces the free pyrimidine, orotic acid. This enzyme is located on the outer face of the inner mitochondrial membrane, in contrast to the other enzymes which are cytosolic. Note the contrast with purine synthesis in which a nucleotide is formed first while pyrimidines are first synthesized as the free base. • Salvaging Purines As a salvage process though, we are dealing with purines. There are two enzymes, A-PRT and HGPRT. A-PRT is not very important because we generate very little adenine. (Remember that the catabolism of adenine nucleotides and nucleosides is through inosine). HG-PRT, though, is exceptionally important and it is inhibited by both IMP and GMP. This enzyme salvages guanine directly and adenine indirectly. Remember that AMP is generated primarily from IMP, not from free adenine • Lesch-Nyhan Syndrome • HG-PRT is deficient in the disease called Lesch-Nyhan Syndrome, a severe neurological disorder whose most blatant clinical manifestation is an uncontrollable self-mutilation. Lesch-Nyhan patients have very high blood uric acid levels because of an essentially uncontrolled de novo synthesis. (It can be as much as 20 times the normal rate). There is a significant increase in PRPP levels in various cells and an inability to maintain levels of IMP and GMP via salvage pathways. Both of these factors could lead to an increase in the activity of the amidotransferase. • Purine Catabolism • The end product of purine catabolism in man is uric acid. Other mammals have the enzyme urate oxidase and excrete the more soluble allantoin as the end product. Man does not have this enzyme so urate is the end product for us. Uric acid is formed primarily in the liver and excreted by the kidney into the urine. • Bases to Uric Acid • Both adenine and guanine nucleotides converge at the common intermediate xanthine. Hypoxanthine, representing the original adenine, is oxidized to xanthine by the enzyme xanthine oxidase. Guanine is deaminated, with the amino group released as ammonia, to xanthine. If this process is occurring in tissues other than liver, most of the ammonia will be transported to the liver as glutamine for ultimate excretion as urea. • Xanthine, like hypoxanthine, is oxidized by oxygen and xanthine oxidase with the production of hydrogen peroxide. In man, the urate is excreted and the hydrogen peroxide is degraded by catalase. Xanthine oxidase is present in significant concentration only in liver and intestine. The pathway to the nucleosides, possibly to the free bases, is present in many tissues. • Gouts and Hyperuricemia • Both undissociated uric acid and the monosodium salt (primary form in blood) are only sparingly soluble. The limited solubility is not ordinarily a problem in urine unless the urine is very acid or has high [Ca2+]. [Urate salts coprecipitate with calcium salts and can form stones in kidney or bladder.] A very high concentration of urate in the blood leads to a fairly common group of diseases referred to as gout. The incidence of gout in this country is about 3/1000. • Gout is a group of pathological conditions associated with markedly elevated levels of urate in the blood (3-7 mg/dl normal). Hyperuricemia is not always symptomatic, but, in certain individuals, something triggers the deposition of sodium urate crystals in joints and tissues. In addition to the extreme pain accompanying acute attacks, repeated attacks lead to destruction of tissues and severe arthritic-like malformations. The term gout should be restricted to hyperuricemia with the presence of these tophaceous deposits. • Urate in the blood could accumulate either through an overproduction and/or an underexcretion of uric acid. In gouts caused by an overproduction of uric acid, the defects are in the control mechanisms governing the production of - not uric acid itself - but of the nucleotide precursors. The only major control of urate production that we know so far is the availability of substrates (nucleotides, nucleosides or free bases). • One approach to the treatment of gout is the drug allopurinol, an isomer of hypoxanthine. • Allopurinol is a substrate for xanthine oxidase, but the product binds so tightly that the enzyme is now unable to oxidized its normal substrate. Uric acid production is diminished and xanthine and hypoxanthine levels in the blood rise. These are more soluble than urate and are less likely to deposit as crystals in the joints. Another approach is to stimulate the secretion of urate in the urine. • Pyrimidine Catabolism • In contrast to purines, pyrimidines undergo ring cleavage and the usual end products of catabolism are beta-amino acids plus ammonia and carbon dioxide. Pyrimidines from nucleic acids or the energy pool are acted upon by nucleotidases and pyrimidine nucleoside phosphorylase to yield the free bases. The 4-amino group of both cytosine and 5-methyl cytosine is released as ammonia. • Ring Cleavage • In order for the rings to be cleaved, they must first be reduced by NADPH. Atoms 2 and 3 of both rings are released as ammonia and carbon dioxide. The rest of the ring is left as a beta-amino acid. Beta-amino isobutyrate from thymine or 5-methyl cytosine is largely excreted. Beta-alanine from cytosine or uracil may either be excreted or incorporated into the brain and muscle dipeptides, carnosine (his-beta-ala) or anserine (methyl his-beta-ala).