* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CH402 Asymmetric catalytic reactions Prof M. Wills
Marcus theory wikipedia , lookup
George S. Hammond wikipedia , lookup
Elias James Corey wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Discodermolide wikipedia , lookup
Polythiophene wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
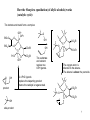
Cracking (chemistry) wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Stille reaction wikipedia , lookup
Aldol reaction wikipedia , lookup
Kinetic resolution wikipedia , lookup
Ene reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Petasis reaction wikipedia , lookup
Hydrogenation wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
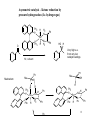
Asymmetric hydrogenation wikipedia , lookup
Hydroformylation wikipedia , lookup
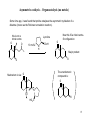
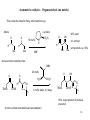
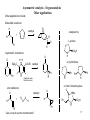
CH402 Asymmetric catalytic reactions Prof M. Wills Think about chiral centres. How would you make these products? H2N H H OH CO2H Ph Ph H OH NMe2 H R1 O H Ph EtO R2 Ph O Think about how you would make them in racemic form first, then worry about the asymmetric 1 versions! What does a catalyst need to be able to provide in a catalytic version? Examples of reactions which form chiral centres Hydrogenation of C=C, C=O, C=N bonds: R 3 4 R H2 gas 2 R1 R catalyst R H 4 R3 2 R1 R O R2 R1 H R2 R1 R R1 R i) BH3 2 R ii) H2O2 R4 OH R3 reducing agent NHR R2 R1 H Epoxidation of C=C bonds: Hydroboration of C=C bonds: 3 R2 R1 H NR 4 OH reducing agent 4 R R3 RCO3H R4 R3 O R2 R1 H R1 R2 R1 R2 2 Examples of reactions which form chiral centres, cont… Hydrocyanation of C=O bonds: Dihydroxylation of C=C bonds: 4 R 3 R 2 R1 R i) OsO4 ii) hydrolysis R4 OH R3 R2 OH R1 O R1 R3 R2 CH2=CH2 catalyst R2 R1 HCN R2 CN R1 Addition of Grignard reagent to C=O bonds: Hydrovinylation of C=C bonds: R4 OH R2 R1 OH O R3 R4 R1 R2 i) RMgBr ii) acid workup R2 R1 R H 3 Examples of reactions which form chiral centres, cont. 2… Enolate alkylation: R3 O R-X 2 R1 Aldol reaction: R3 O R2 R1 R R O R3 R1 R2 R6 R5 R8 R7 R1 (aldehyde) R1 R3 (three chiral centres) H OH R2 R Hydroformylation of C=C bonds: Diels-Alder (cycloaddition): 4 R6 R RCHO Enolate Enolate (formed by ketone deprotonation) R5 O R3 R4 R3 R2 R2 R1 R8 R7 Four chiral centres H R4 R1 And many, many more…. R3 R2 CO, H2 catalyst O R3 R4 R2 R1 H 4 What properties are required of an asymmetric catalyst? Turnover, rate enhancement, selectivity The catalyst must recognise the reagents, accelerate the reaction, direct the reaction to one face of a substrate and release the product: Catalyst recycled catalyst + + recognition substrate 1 reaction catalyst (a bond forms) substrate 2 catalyst + release Product! 5 Asymmetric epoxidation of alkenes (1980s) 4 R R3 R4 RCO3H R3 Mechanism? Could you modify this in an asymmetric manner? O R1 R1 R2 R2 Sharpless discovered that a combination of diethyl tartrate, titanium isopropoxide and a peroxide. But it requires an allylic alcohol as substrate. The oxidant is used stoichiometrically (i.e. you need one equivalent), but the titanium and tartrate are used in catalytic amounts (ca. 5 mol%). t-butyl peroxide (oxygen source) O O H Ti(OiPr)4 (metal for complex formation) OH HO HO O CO2Et (+)-diethyl tartrate (source of CO2Et chirality) OH 70-90% yield, >90% e.e. The (-)-diethyl tartrate gives the opposite enantiomer. 6 How the Sharpless epoxidation (of allylic alcohols) works (catalytic cycle): The tartrate and metal form a complex: EtO2C O OiPr OiPr O OH Ti EtO2C O Ti O CO2Et OH O OH product O OiPr CO2Et The substrate and oxidant replace two OiPr ligands. O O Ti PrOi O 2 x iPrO ligands replace the departing product hence the catalyst is regenerated. CO2Et O O CO2Et Ti The oxygen atom is directed to the alkene. The alkene is above the peroxide. Ti O O OH O O O CO2Et O O CO2Et Ti side-product 7 Asymmetric epoxidation of alkenes using Mn/Salen complexes (Jacobsen epoxidation): The iodine reagent transfers its oxygen atom to Mn, then the Mn tranfers in to the alkene in a second step. The chirality of the catalyst controls the absolute configuration. Advantage? You are not limited to allylic alcohols. catalyst 5 mol% H H N N Mn But O tBu O tBu But O I O (hypervalnet iodine reagent) Source of oxygen. 8 Asymmetric hydrogenation for the synthesis of amino acids: Addition of hydrogen to an acylamino acrylate results in formation of an amine acid precursor. <1 mol% Ph Rh. catalyst O HO2C Ph N H HO2C H H2 -acylamino acrylate O S N H N-acylated amine acid. The combination of an enantiomerically-pure (homochiral) ligand with rhodium(I) results in formation of a catalyst for asymmetric reactions. MeO P P .. .. OMe RR-DiPAMP = a homochiral ligand OMe P S Rh P S MeO DiPAMP coordinated to Rh(I) 9 Asymmetric catalysis - hydrogenation Rh-diphosphine complexes control asymmetric induction by controlling the face of the alkene which attaches to the Rh. Hydrogen is transferred, in a stepwise manner, from the metal to the alkene. The intermediate complexes are diastereoisomers of different energy. Rh/DiPAMP OMe OMe Ph P HO2C Rh Ph P O OMe P O Rh N H P CO2H N H More stable, but less reactive complex OMe Less stable, but more reactive leads to product H2 Ph O H H S N H H CO2H Using Rh(DIPAMP) complexes, asymmetric reductions may be achieved in very high enantioselectivity. 10 Asymmetric catalysis - hydrogenation Other chiral diphosphines are not chiral at P, but contain a chiral backbone which ‘relays’ chirality to conformation of the arene rings. PPh2 Rh/Diphosphine complex PPh2 H PPh2 S-BINAP P PPh2 H H O PPh2 O PPh2 Chiraphos face edge face Rh P edge H DIOP 11 Asymmetric catalysis – Ketone reduction The reduction of a ketone to a secondary alcohol is a perfect reaction for asymmetric catalysis: HO H O i) Borane (BH3), oxazaborolidine catalyst ii) hydrolysis (work up) Ph Oxazaborolidine catalyst: H Ph Ph O N B Concave molecule hydride directed to one face. O How it works: N H Ph B Me O B H H H Me 12 Asymmetric catalysis – Ketone reduction by pressure hydrogenation (I.e. hydrogen gas) Ph2 P H H2 N Ph N H2 Ph Ru P Ph2 O H Very high e.e. from very low catalyst loadings H2 , solvent Mechanism Ph O Me Ph2 P P Ph2 H Ru H HO H H H N N H2 Ph OH Me H Ph Ph H2 Ph2 P P Ph2 Ru H N H N H2 Ph Ph 13 Asymmetric catalysis – Isomerisation Ph2 P Rh PPh2 H NMe2 NMe2 [Rh/S-BINAP] Isomerisation (not a reduction!) H H ZnBr2 OH (-)-menthol then H2, Ni cat (to reduce alkene) H O R-citronellal, 96-99% e.e. 14 Asymmetric catalysis – Organocatalysis (no metals) Some time ago, it was found that proline catalyses the asymmetric cyclisation of a diketone (known as the Robinson annelation reaction). this is not a chiral centre O Now this IS a chiral centreS configuration L-proline O 10 mol%: N H CO2H O Major product O O The enantiomeric compound is: Mechanism is via: O O N O HO2C O 15 Asymmetric catalysis – Organocatalysis (no metals) This is now the basis for many other reactions e.g.: L-proline Aldols: O O 10 mol%: N H H H Me Me 90% yield O CO2H OH 4:1 anti:syn H Me DMF Me anti product e.e.: 99% and even more complex ones: OtBu 20 mol% O H2N H TBSO O O OTBS CO2H O O O OH 3 mol% water, rt 2 days. TBSO OTBS O 68%, major product: D-fructose precursor (it turns out that most amines act as catalysts!) 16 Asymmetric catalysis – Organocatalysis Other applications Other applications include: Diels-Alder reactions: O catalyst catalysed by: + H R L-proline O R H Asymmetric reductiions: O H H O Ph N H Ph or pyrrolidines: CO2Et catalyst EtO + 2C N H H (Hantzsch esterhydride source) H O catalyst + R O O H R Can you work out the mechanisms? Ph Ph N H or other N-heterocycles: O NMe and oxidations: O CO2H N H H O R Ph N H CO2H 17 Ph Asymmetric catalysis – Enolate alkylation The reaction proceeds via a complex in which the catalyst and the enolate are bound by a hydrogen bond (at least, that's the theory): Cl O 10 mol% (i.e. 01 eq.) Catalyst (below), 50% NaOH-toluene Cl CH3Cl MeO Cl O Cl 98% yield 94% e.e. MeO H O H several steps N Catalyst: N Cl Cl MeO The enolate is formed by deprotonation by hydroxide. O CF3 Cl O Cl Enolate is methylated on the front face (as illustrated) O CO2H indacrinone 18 Asymmetric catalysis – Enolate alkylation for synthesis of amino acids. By using an amino acid precursor with a relatively low pKa, it is possible to alkylate under relatively mild conditions: Ph Ph OtBu Ph N O Ph 10 mol% Catalyst (below), 50% NaOH - toluene PhCH2Br Ph full conversion OtBu 90-95% e.e. N O several steps H O H Catalyst: N N Ph O Think about the mechanism and the enantiocontrol. H3N O 19 Asymmetric catalysis – Addition to an aldehyde (C-C bond forming reaction) – for interest only. HO Et O H Et2Zn, toluene (solvent) H See table for results (-)-DAIB (see below) Results: NMe2 mol% DAIB used Yield (relative to aldehyde) E.e. (-)-DAIB 0 (i.e. none) 0% - (two pictures of the same molecule) 2 (0.02 eq.) 97% 98 100 (1.0 eq.) 0% - OH NMe2 OH How come a little bit of amino alcohol catalyses the reaction, but a lot of it doesn't? 20