* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 17_Oxidative decarboxylation of pyruvate and Krebs cycle
Light-dependent reactions wikipedia , lookup
Photosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Mitochondrion wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Microbial metabolism wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Electron transport chain wikipedia , lookup
Butyric acid wikipedia , lookup
Biosynthesis wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
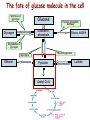
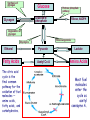
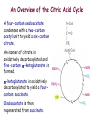
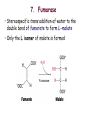
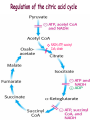
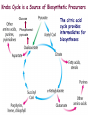
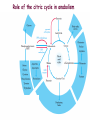
Oxidative decarboxylation of pyruvate and Krebs cycle OXIDATIVE DECARBOXYLATION OF PYRUVATE Matrix of the mitochondria contains pyruvate dehydrogenase complex The fate of glucose molecule in the cell Synthesis of glycogen Glucose Glucose-6phosphate Glycogen Pentose phosphate pathway Ribose, NADPH Degradation of glycogen Gluconeogenesis Glycolysis Ethanol Pyruvate Acetyl Co A Lactate OXIDATIVE DECARBOXYLATION OF PYRUVATE Only about 7 % of the total potential energy present in glucose is released in glycolysis. Glycolysis is preliminary phase, preparing glucose for entry into aerobic metabolism. Pyruvate formed in the aerobic conditions undergoes conversion to acetyl CoA by pyruvate dehydrogenase complex. OXIDATIVE DECARBOXYLATION OF PYRUVATE Pyruvate dehydrogenase complex is a bridge between glycolysis and aerobic metabolism – citric acid cycle Pyruvate dehydrogenase complex and enzymes of cytric acid cycle are located in the matrix of mitochondria Entry of Pyruvate into the Mitochondrion Pyruvate freely diffuses through the outer membrane of mitochondria through the channels formed by transmembrane proteins porins. Pyruvate translocase, protein embedded into the inner membrane, transports pyruvate from the intermembrane space into the matrix in symport with H+ and exchange (antiport) for OH-. Conversion of Pyruvate to Acetyl CoA • Pyruvate dehydrogenase complex (PDH complex) is a multienzyme complex containing 3 enzymes, 5 coenzymes and other proteins. Pyruvate dehydrogenase complex is giant, with molecular mass ranging from 4 to 10 million daltons. Electron micrograph of the pyruvate dehydrogenase complex from E. coli. Pyruvate dehydrogenase complex Enzymes: E1 = pyruvate dehydrogenase E2 = dihydrolipoyl acetyltransferase E3 = dihydrolipoyl dehydrogenase Pyruvate dehydrogenase complex Coenzymes: TPP (thiamine pyrophosphate), lipoamide, HS-CoA, FAD+, NAD+. TPP is a prosthetic group of E1; lipoamide is a prosthetic group of E2; and FAD is a prosthetic group of E3. The building block of TPP is vitamin B1 (thiamin); NAD – vitamin B5 (nicotinamide); FAD – vitamin B2 (riboflavin), HS-CoA – vitamin B3 (pantothenic acid), lipoamide – lipoic acid Pyruvate dehydrogenase complex is a classic example of multienzyme complex Overall reaction of pyruvate dehydrogenase complex The oxidative decarboxylation of pyruvate catalized by pyruvate dehydrogenase complex occurs in five steps. Aerobic cells use a metabolic wheel – the citric acid cycle – to generate energy by acetyl CoA oxidation The Citric Acid Cycle Synthesis of glycogen Glucose Pentose phosphate pathway Glucose-6phosphate Glycogen Ribose, NADPH Degradation of glycogen Gluconeogenesis Glycolysis Ethanol Fatty Acids The citric acid cycle is the final common pathway for the oxidation of fuel molecules — amino acids, fatty acids, and carbohydrates. Pyruvate Lactate Acetyl Co A Amino Acids Most fuel molecules enter the cycle as acetyl coenzyme A. Names: The Citric Acid Cycle Tricarboxylic Acid Cycle Krebs Cycle In eukaryotes the reactions of the citric acid cycle take place inside mitochondria Hans Adolf Krebs. Biochemist; born in Germany. Worked in Britain. His discovery in 1937 of the ‘Krebs cycle’ of chemical reactions was critical to the understanding of cell metabolism and earned him the 1953 Nobel Prize for Physiology or Medicine. An Overview of the Citric Acid Cycle A four-carbon oxaloacetate condenses with a two-carbon acetyl unit to yield a six-carbon citrate. An isomer of citrate is oxidatively decarboxylated and five-carbon -ketoglutarate is formed. -ketoglutarate is oxidatively decarboxylated to yield a fourcarbon succinate. Oxaloacetate is then regenerated from succinate. An Overview of the Citric Acid Cycle Two carbon atoms (acetyl CoA) enter the cycle and two carbon atoms leave the cycle in the form of two molecules of carbon dioxide. Three hydride ions (six electrons) are transferred to three molecules of NAD+, one pair of hydrogen atoms (two electrons) is transferred to one molecule of FAD. The function of the citric acid cycle is the harvesting of high-energy electrons from acetyl CoA. 1. Citrate Synthase • Citrate formed from acetyl CoA and oxaloacetate • Only cycle reaction with C-C bond formation • Addition of C2 unit (acetyl) to the keto double bond of C4 acid, oxaloacetate, to produce C6 compound, citrate citrate synthase 2. Aconitase • Elimination of H2O from citrate to form C=C bond of cis-aconitate • Stereospecific addition of H2O to cis-aconitate to form isocitrate aconitase aconitase 3. Isocitrate Dehydrogenase • Oxidative decarboxylation of isocitrate to a-ketoglutarate (a metabolically irreversible reaction) • One of four oxidation-reduction reactions of the cycle • Hydride ion from the C-2 of isocitrate is transferred to NAD+ to form NADH • Oxalosuccinate is decarboxylated to a-ketoglutarate isocitrate dehydrogenase isocitrate dehydrogenase 4. The -Ketoglutarate Dehydrogenase Complex • Similar to pyruvate dehydrogenase complex • Same coenzymes, identical mechanisms E1 - a-ketoglutarate dehydrogenase (with TPP) E2 – dihydrolipoyl succinyltransferase (with flexible lipoamide prosthetic group) E3 - dihydrolipoyl dehydrogenase (with FAD) -ketoglutarate dehydrogenase 5. Succinyl-CoA Synthetase • Free energy in thioester bond of succinyl CoA is conserved as GTP or ATP in higher animals (or ATP in plants, some bacteria) • Substrate level phosphorylation reaction + Succinyl-CoA Synthetase GTP + ADP GDP + ATP HS- 6. The Succinate Dehydrogenase Complex • Complex of several polypeptides, an FAD prosthetic group and iron-sulfur clusters • Embedded in the inner mitochondrial membrane • Electrons are transferred from succinate to FAD and then to ubiquinone (Q) in electron transport chain • Dehydrogenation is stereospecific; only the trans isomer is formed Succinate Dehydrogenase 7. Fumarase • Stereospecific trans addition of water to the double bond of fumarate to form L-malate • Only the L isomer of malate is formed Fumarase 8. Malate Dehydrogenase Malate is oxidized to form oxaloacetate. Malate Dehydrogenase Stoichiometry of the Citric Acid Cycle Two carbon atoms enter the cycle in the form of acetyl CoA. Two carbon atoms leave the cycle in the form of CO2 . Four pairs of hydrogen atoms leave the cycle in four oxidation reactions (three molecules of NAD+ one molecule of FAD are reduced). One molecule of GTP, is formed. Two molecules of water are consumed. Stoichiometry of the Citric Acid Cycle 9 ATP (2.5 ATP per NADH, and 1.5 ATP per FADH2) are produced during oxidative phosphorylation 1 ATP is directly formed in the citric acid cycle 1 acetyl CoA generates approximately 10 molecules of ATP Functions of the Citric Acid Cycle • Integration of metabolism. The citric acid cycle is amphibolic (both catabolic and anabolic). The cycle is involved in the aerobic catabolism of carbohydrates, lipids and amino acids. Intermediates of the cycle are starting points for many anabolic reactions. • Yields energy in the form of GTP (ATP). • Yields reducing power in the form of NADH2 and FADH2. Regulation of the Citric Acid Cycle • Pathway controlled by: (1) Allosteric modulators (2) Covalent modification of cycle enzymes (3) Supply of acetyl CoA (pyruvate dehydrogenase complex) Regulation of the Citric Acid Cycle Three enzymes have regulatory properties - citrate synthase (is allosterically inhibited by NADH, ATP, succinyl CoA, citrate – feedback inhibition) - isocitrate dehydrogenase (allosteric effectors: (+) ADP; (-) NADH, ATP. Bacterial ICDH can be covalently modified by kinase/phosphatase) --ketoglutarate dehydrogenase complex (inhibition by ATP, succinyl CoA and NADH Regulation of the citric acid cycle - NADH, ATP, succinyl CoA, citrate Krebs Cycle is a Source of Biosynthetic Precursors Glucose Phosphoenolpyruvate The citric acid cycle provides intermediates for biosyntheses Role of the citric cycle in anabolism