* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Digestion of Proteins
Expression vector wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Signal transduction wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Peptide synthesis wikipedia , lookup
Interactome wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Magnesium transporter wikipedia , lookup
Catalytic triad wikipedia , lookup
Genetic code wikipedia , lookup
Phosphorylation wikipedia , lookup
Human digestive system wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Western blot wikipedia , lookup
Metalloprotein wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Biosynthesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
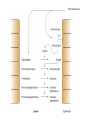
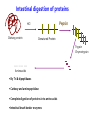
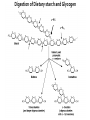
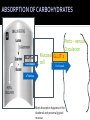
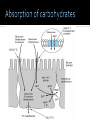
Explain the process of digestion & absorption of proteins and carbohydrates in the GIT. Dr. Mohammed Vaseem Assistant Professor Biochemistry Protein Peptide Bond Polysaccharide Oligosaccharide Disaccharide Starch Glycogen Sucrose Maltose Lactose Hydrolase Digestion of Proteins H2O R1 R2 R1-COOH + R2-NH2 Peptide bond – Partial Double bond - Stable Hydrolysis extremely slow (absence of catalyst 10 100 years) In vitro – 6N HCl, 100⁰ C for 24 – 36 hrs. Proteolytic enzymes – nucleophilic attack on carbonyl group Digestion of proteins By the action of peptidases Endo peptidases – Acts on interior peptide bonds NH2 ------------------------------------------------ COOH Eg: Typsin, Chymotrypsin, Rennin Exo peptidases – Acts on terminal peptide bonds Carboxy peptidase NH2 ----------------------------------------------- COOH Amino peptidase N terminal C - terminal Exopeptidases Endopeptides Digestion of proteins Proteins HCl Denaturation Pepsin Polypeptides Trypsin Chymotrypsin Small Peptides Amino peptidase Carboy peptidase Dipeptidases Tripeptidases Amino acids Gastric digestion of proteins Proteolytic enzymes are secreted as zymogens (inactive) Only Activated at specific site of action Gastrin – gastric mucosa – Stimulates gastric secretion HCl (Parietal cells) denature the dietary protein HCl activates pepsin (pH = 1-2) HCl Pepsinogen (42KDa) (Chief cells) Removal of N-terminal 44 AA Pepsin (34KDa) Gastric digestion contd… Pepsin • Proteins Proteoses & Peptones • Rennin helps in the digestion of milk in infants • Casein Rennin Paracaseinate (partially digested) Pepsin HCl Dietary protein Acted on by pepsin Denatured Protein Peptones & Proteoses Pancreatic digestion of Proteins Entry of acidic food stimulate intestinal hormones Cholecystokinin & Secretin – Intestinal hormones stimulates secretion of pancreatic juice Contains alkaline bicarbonate (pH 8.0) & Zymogens Intestinal digestion of proteins Pepsin HCl Dietary protein Denatured Protein Trypsin Chymotrypsin -- - - - - - - - - Aminoacids • By Tri & dipeptidases • Carboxy and aminopeptidase • Complete digestion of proteins in to amino acids •Intestinal brush border enzymes Proteolytic enzymes are highly specific in action Enzyme Bond hydrolysis Pepsin Phe, Tyr, Trp, Met Trypsin Arg, Lys Chymotrypsin Phe, Tyr, Trp, Val, Leu, Met Elastase Ala, Gly, Ser Carboxypeptidase A Ala, Ile, Leu, Val Carboxypeptidase B Arg, Lys When the C-terminal Amino acid is as listed Most of the proteolytic enzymes are serine proteases Catalytic traid – Histidine , Asp & Ser (Chymotrypsin, Typsin & Elastase Other proteases 1) Cysteine proteases – Papain 2) Aspartyl proteases – Renin 3) Metallo proteases (mostly Zn) - Carboxy peptidase A Protease inhibitors Natural Pancreatic trypsin inhibitor – inhibits trypsin α1 - Anti –trypsin – Inhibits elastase Absorption of Amino acids Mainly in small intestine (duodenum and jejunum) Free amino acids are absorbed by active transport Active Transport Sodium dependant secondary active transport system Five different transport system (neutral, basic, acidic, imno acids & beta amino acids) Di- and tri-peptides are absorbed by a proton linked active transport (hydrolysed in the cytoplasm) Role of Tri-peptide - glutathione (Miester cycle) Hartnup’s disease – defect in absorption •Intestine, Brain, Kidney •Mainly neutral AA Largest source of calories in the average diet Usually constitute 40 to 45% of our caloric intake The plant starches amylopectin and amylose, which are present in grains, tubers, and vegetables, constitute approximately 50 to 60% of the carbohydrate calories consumed. These starches are polysaccharides, containing 10,000 to 1 million glucosyl units. Amylose, the glucosyl residues form a straight chain linked via -1,4 glycosidic bonds; Amylopectin, the -1,4 chains contain branches connected via -1,6 glycosidic bonds The other major sugar found in fruits and vegetables is sucrose, a disaccharide of glucose and fructose Sucrose and small amounts of the monosaccharides glucose and fructose are the major natural sweeteners found in fruit, honey, and vegetables. The portion of the dietary carbohydrate that cannot be digested by human intestinal enzymes It Is composed principally of plant polysaccharides cellulose, lignins and pectins Contain very little carbohydrate except for small amounts of glycogen (which has a structure similar to amylopectin) The major dietary carbohydrate of animal origin is lactose, a disaccharide composed of glucose and galactose found exclusively in milk and milk products • Carbohydrate digestion begins in the mouth • No Enzymatic Hydrolysis occurs in the Stomach • There is participation of Pancreas • Final steps are catalyzed by small intestinal enzymes Dietary polysaccharides and disaccharides are converted to monosaccharides by glycosidases Enzymes that hydrolyze the glycosidic bonds between the sugars. All of these enzymes exhibit some specificity for the sugar, the glycosidic bond ( or ), and the number of saccharide units in the chain. Undigested carbohydrates enter the colon, where they may be fermented by bacteria. Digestion of Dietary starch and Glycogen 16 14 • DISACCHARIDES ARE CLEAVED TO MONOSACCS. BY SPECIFIC ENZYMES Lactose + H2O D-glucose + D-galactose Lactase Sucrose + H2O Sucrase = Invertase D-glucose + D-fructose Maltose + H2O 2 D-glucose Maltase Trehalose + H2O 2 D- glucose Trehalase Isomaltose + H2O 2 D- glucose Isomaltase Glucoamylase: exoglucosidase that is specific for the –1,4 bonds between glucosyl residues begins at the nonreducing end of a polysaccharide or limit dextrin, and sequentially hydrolyzes the bonds to release glucose monosaccharides ENZYMES ARE SPECIFIC FOR THE SUBSTRATE • Amylase Acts on 1 4 glycosidic linkage • Maltase Acts on maltose and maltotriose (14 ) • Iso-maltase Acts on -16 glycosidic linkage • Lactase Acts on -14 glycosidic linkage • Sucrase Acts on 12 glycosidic linkage • Trehalase Acts on 11 glycosidic linkage • β - GLYCOSIDASE COMPLEX (LACTASE-GLUCOSYLCERAMIDASE) What is dietary fibre? ABSORPTION OF CARBOHYDRATES GLUT 2 Facilitated Mucosal GLUT 2 cell Facilitated Porto - venous Circulation 2 ⁰ Active fructose Most absorption happens in the duodenal and proximal jejunal mucosa MALABSORBTION OF CARBOHYDRATRES Enzyme deficiency Primary mutation in transporter Proteins Secondary Following intestinal disease Following disease of pancreas