* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download New functions of the Drosophila rhomboid gene
Minimal genome wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Genetic engineering wikipedia , lookup
Public health genomics wikipedia , lookup
Ridge (biology) wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
History of genetic engineering wikipedia , lookup
Oncogenomics wikipedia , lookup
X-inactivation wikipedia , lookup
Genome evolution wikipedia , lookup
Gene desert wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Quantitative trait locus wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Genomic imprinting wikipedia , lookup
Dominance (genetics) wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Genome (book) wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Designer baby wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Microevolution wikipedia , lookup
Gene expression profiling wikipedia , lookup
Gene expression programming wikipedia , lookup
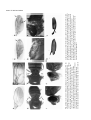
Development 120, 2329-2338 (1994) Printed in Great Britain © The Company of Biologists Limited 1994 2329 New functions of the Drosophila rhomboid gene during embryonic and adult development are revealed by a novel genetic method, enhancer piracy Rebekka Noll†, Mark A. Sturtevant, Raghava R. Gollapudi and Ethan Bier* Department of Biology and Center for Molecular Genetics, University of California, San Diego, La Jolla, CA 92093, USA *Author for correspondence †Present address: Department of Cellular and Developmental Biology, Harvard Medical School, Boston Massachusetts 02115, USA SUMMARY Localized expression of the Drosophila rhomboid (rho) gene has been proposed to hyperactivate EGF-Receptor signaling in specific cells during development of the embryo and adult. In this report we use a novel transposon based genetic method, enhancer piracy, to drive ectopic expression of a rho cDNA transgene by endogenous genomic enhancers. Many enhancer piracy transposon-rho insertions cause dominant phenotypes, over half of which cannot be duplicated by ubiquitous expression of rho. Genetic interactions between various dominant enhancer piracy alleles and mutations in the EGF-R/RAS signaling pathway indicate that many of these novel phenotypes result from ectopic activation of EGF-R signaling. Patterned mis-expression of the rho cDNA transgene correlates in several cases with localized dominant enhancer piracy phenotypes. Enhancer piracy lines reveal an unanticipated role for rho in imaginal disc formation and provide the first evidence that mis-expression of rho is sufficient for converting entire intervein sectors into veins. Enhancer piracy may prove to be a general strategy for obtaining dominant alleles of a gene of interest in diverse insects, worms, plants, and potentially in vertebrates such as mice and fish. INTRODUCTION signaling as ubiquitous expression of rho from a heat shock construct generates several dominant phenotypes consistent with activating the EGF-R signaling pathway in inappropriate cells. Various phenotypes have been recovered in heat shock experiments depending on the stage at which heat induction is administered (Freeman et al., 1992; Sturtevant et al., 1993; Ruohola-Baker et al., 1993; J.W. O’Neill and E. Bier, unpublished data; M. Sturtevant and E. Bier, unpublished data – see Table 1 in this study), confirming the expectation that localized expression of rho is important for targeting EGF-R signaling to appropriate cells at a variety of developmental stages. One difficulty in analyzing the role of ectopic rho expression during many developmental stages such as embryogenesis is that rho is often expressed in many different cell types simultaneously. This complicates interpretation of resulting heat shock phenotypes since mixtures of supernumerary and missing cell types are expected. One solution to this problem is to provide ectopic rho expression in a limited number of cells. To this end we employed enhancer piracy, a novel genetic approach similar to that used in the enhancer trapping method. Enhancer trapping has proved to be a versatile and powerful method for identifying genes based on the activity of neighboring enhancer elements (O’Kane and Gehring, 1987; Bier et al., 1989; Bellen et al., 1989). Enhancers functioning at a distance can activate the expression of a reporter gene carried on a transposon inserted at neighboring chromosomal The Drosophila rhomboid (rho) gene is expressed in a complex localized pattern during embryogenesis and imaginal disc development and is required for the differentiation of many of the primordia in which it is expressed (Mayer and NüssleinVolhard, 1988; Bier et al., 1990; Sturtevant et al., 1993). Several lines of evidence suggest that one important function of rho is to participate as an accessory molecule to promote signaling through the EGF-Receptor (EGF-R)/RAS pathway. The clearest evidence that rho functions to amplify EGF-R signaling derives from the detailed similarity of embryonic loss of function phenotypes for rho (Mayer and Nüsslein-Volhard, 1988; Bier at al., 1990), Egf-r (Clifford and Schüpbach, 1992; Raz and Shilo, 1992, 1993), and spitz (Mayer and NüssleinVolhard, 1988) which encodes an EGF-like ligand (Rutledge et al., 1992); from a wealth of self consistent genetic interactions between rho alleles and mutations in components of the EGF-R signaling pathway (Sturtevant et al., 1993); and from evidence that rho acts upstream of Egf-r in oogenesis (Ruohola-Baker et al., 1993). An interesting feature of regulation of EGF-R signaling by rho is that the ligand and receptor are ubiquitously expressed during embryogenesis and imaginal disc development, while rho expression is highly localized and is mostly confined to cells requiring the activity of EGF-R. Localized expression of rho is critical for restricting EGF-R Key words: rhomboid, Egf-r, Drosophila, enhancer piracy, enhancer trapping, transposon, dominant allele, genetic interaction 2330 R. Noll and others sites (Bier et al., 1989; Wilson et al., 1989). A remarkable feature of enhancer trap screens is that over half of the sites of random transposon insertion express the reporter gene in some tissue-specific or spatially localized pattern (O’Kane and Gehring, 1987; Bier et al., 1989; Bellen et al., 1989). We reasoned that the same genetic strategy could be applied to driving the localized expression of an active gene of interest in place of the passive reporter gene. Thus, mobilization of an enhancer trap-like vector carrying a cDNA of interest should result in transposon insertions near endogenous genomic enhancers which could drive localized expression of the cDNA. Since ubiquitous expression of rho yields dominant phenotypes at several stages of development (Sturtevant et al., 1993) and because several of the original HS-rho transformants have dominant constitutive extra wing vein phenotypes (Sturtevant et al., 1993), we expected that a more systematic attempt to generate dominant alleles might reveal new functions of rho. Furthermore, such dominant phenotypes could serve as valuable genetic tools for isolating second site suppressors or enhancers of the dominant HS-rho phenotypes. In this paper we use enhancer piracy to generate a variety of dominant rho alleles. Over half of these enhancer piracy phenotypes cannot be mimicked by ubiquitous expression of rho, although we recovered enhancer piracy insertion lines with dominant phenotypes typical of all those observed following various heat shock treatments of HS-rho flies. Some of these new dominant rho alleles provide important insights into the developmental function of rho. In several cases we determined that enhancer piracy lines exhibiting localized defects express the rho transgene in a pattern corresponding to the primordia for those affected structures. We discuss the potential of enhancer piracy as a versatile general method for obtaining dominant alleles of Drosophila genes and the possibility of employing similar strategies in other organisms. MATERIALS AND METHODS Fly stocks All genetic markers and chromosome balancers used are described in Lindsley and Grell (1968) and Lindsley and Zimm (1992). Crosses to generate transpositions from the X chromosome to autosomes using a genomic source of transposase (∆2-3) were performed as described in Bier et al. (1989). Several strains of flies carrying X chromosome insertions of the HS-rho P-element construct (Sturtevant et al., 1993) were tested in pilot studies according to the scheme depicted in Fig. 1 to determine which line gave the optimal transposition frequency. The line with the highest transposition frequency (60% of vial crosses using 2 HS-rho; ∆2-3 Sb/+ males × 5 yw females gave at least one jump) was used to generate the majority of lines generated for this study. Other stocks were obtained from the Bloomington, Indiana Drosophila Stock Center and the Bowling Green, Ohio Stock Center. Mounting fly wings Wings from adult flies were dissected in isopropanol and mounted in Canadian Balsam mounting medium (Gary’s Magic Mount) following the protocol of Lawrence et al. (in Roberts, 1986). Mounted wings were photographed under Nomarski optics with a 4× lens on a compound microscope. Alternatively, whole flies or portions of flies were photographed through a dissection microscope. In situ hybridization to whole-mount embryos or discs In situ hybridization to whole-mount discs and embryos was performed using digoxigenin-labeled RNA probes (BoehringerMannheim, 1093 657) according to the method Tautz and Pfeiffle (1989), using 4 µg/ml Proteinase K instead of 40 µg/ml as required for digoxigenin-labeled DNA probes. rho transgene expression was visualized with a 850 bp probe specific to the 3′ transcribed SV40 sequences derived from the HS-rho vector (Sturtevant et al., 1993). No signal is observed when using this 3′ trailer probe on wild-type embryos. Other molecular techniques RNA probe synthesis was performed according to BoehringerMannheim protocols and other cloning techniques followed standard procedures, as described by Maniatis et al. (1982). RESULTS Enhancer piracy is an efficient method for recovering dominant rho phenotypes We mobilized a P-element heat shock vector (hs-CaSpeR; see Bang and Posakony, 1992) carrying the rho protein coding region (HS-rho, see Sturtevant et al., 1993) from the X chromosome to autosomal sites using the genetic scheme depicted in Fig. 1. We chose a heat shock vector for this screen because many fly strains already exist carrying various cDNA inserts in heat shock vectors. As the hs-CaSpeR vector provides high levels of ubiquitous expression following heat shock induction and functions efficiently for generating enhancer piracy lines (see below), it is an excellent vector for both of these purposes and is consequently ideal for mis-expressing a gene of interest. Individual transformant flies were scored for dominant yw ; + Sb 2-3 p[y ] TM6Ubx w p[HS-rho, w + ] Sb ; + + w p[HS-rho, w +] ; w p[HS-rho, w + ] X + 2-3 p[y ] X + yw yw ; + + (white eyes) Transposition to an autosome yw orange eyes yw ; ; p[HS-rho, w +] + + + ; Establish Stocks ; + + p[HS-rho, w + ] X yw yw + Test for dominant sterility 2-3 = p-element transposase (defective for transposition) p[HS-rho, w +] = p-element vector containing the rho coding region under the control of the hsp70 promoter. The vector also contains the dominant white (w+) marker. Fig. 1. Genetic scheme for generating rhomboid enhancer piracy transposon insertions. A single male transformant obtained from the screen was immediately scored for phenotype and was then mated to yw females to confirm that the dominant phenotype was heritable and to establish permanent stocks for interesting phenotypes. Heterozygous females from 113 lines were mated to yw males to determine whether any insertions were dominant female sterile. Enhancer piracy and rhomboid function 2331 Table 1. Enhancer piracy phenotypes due to HS-rho insertions X Y Z X X+Y+Z Observed with heat shock†† 21 108 75 16 1 (−)† (−) (−) 9 1 (−) (−) (−) 14 3 8.5 43.5 30.2 6.5 0.4 8.5 43.5 30.2 15.7 2.0 NA + +/− +/− − No. of lines (248 total) Phenotype Wing None* Weak‡ Moderate§ Strong¶ Serrated % of total lines Thorax Haltereless Shoulderless Duplicated wing 2 0 0 6 6 2 0 6 0 0.8 0 0 3.2 4.8 0.8 − − − Bristle Extra orbitals 1 8 0 0.4 3.6 − Eye Rough eye 2 4 2 0.8 3.2 + Leg Malformed 0 2 2 0 1.6 − Other Female sterile** 1 0 0 0.9 0.9 + X = >50% of individuals show phenotype. Y = <50 % of individuals show phenotype (typically 20-40%). Z = Primary transformant showed phenotype but either died or failed to mate. *None = wild-type wing. †(−) = Wing phenotypes of moderate or less than moderate strength were only scored if highly penetrant. ‡Weak = One or more of: very short extra vein segments, small deltas, and very short thickened vein segments. §Moderate = One or more of: extended thickened veins, long extra vein segments, large and connected deltas, and small blisters. ¶Strong = One or more of: widespread thickened veins, large blisters, and shriveled wings. **113 lines were tested for dominant female sterility. ††Phenotypes that have been observed with heat shock are marked with (+); phenotypes never been recovered with heat shock are marked with (−); and for those marked with (+/−) some but not all of the phenotypes in this category were oberved with heat shock. For example, reproducibly localized blisters (moderate wing - Fig. 2C) and extremely thick veins (strong wing - Fig. 2D) were not recovered in heat shock experiments. NA = not applicable. phenotypes at the time they were recovered and were then mated to yw flies to determine whether the observed phenotypes segregated with the HS-rho vector (marked with w+). The degree of penetrance (i.e. greater or less than 50%) was also determined for lines with phenotypes. The kinds of phenotypes recovered from screening of 248 independent autosomal rho enhancer piracy insertions and the relative frequency of these phenotypes are summarized in Table 1. Many of these phenotypes have not been recovered in exhaustive heat shock induction experiments (Table 1; J.W. O’Neill and E. Bier unpublished data; M. Sturtevant and E. Bier, unpublished data). Enhancer piracy phenotypes reveal novel requirements for localized rho expression Wing phenotypes, including a variety of strengths of excess vein material or blisters, were recovered at high frequency (Fig. 2B-D). This is consistent with previous data suggesting that even low levels of ectopic rho expression during wing development generate excess vein phenotypes (Sturtevant et al., 1993). Some lines exhibited localized wing venation defects, such as segments of thickened veins or small blisters restricted to particular regions of the wing (Fig. 2C). The conversion of complete intervein sectors into vein tissue (between arrows, but not between veins L3 and L4) in the line shown in Fig. 2D reveals that all intervein cells in these sectors of the wing can be induced to assume vein fates, a fact not illumi- nated by heat shock experiments. An interesting category of wing phenotype also not recovered by heat shock is a serrated wing margin. Another class of phenotypes only recovered in these enhancer piracy experiments involves thoracic structures. Tissues derived from haltere and wing discs are affected to varying degrees in the enhancer piracy line shown in Fig. 2FH. The most common defect is a loss of one or both halteres (Fig. 2F). Strongly affected individuals may also lack part of the wing, resulting in the formation of a mirror symmetric double anterior wing (Fig. 2G), or may lack virtually all structures derived from the wing disc which is manifested as a shoulderless phenotype (Fig. 2H). Intriguingly, similar loss of imaginal tissue phenotypes and mirror duplications have recently been reported for various combinations of alleles of the ventro-lateral group gene pointed (Scholz et al., 1993). Various allelic combinations of sna and neighboring genes also result in similar loss of halteres and defective thoracic phenotypes (Ashburner et al., 1990). The basis for the haltereless/shoulderless phenotypes was investigated in two independent enhancer piracy lines. As haltere discs were frequently missing from third instar larvae of these lines, we examined the appearance of the wing and haltere discs during embryogenesis. Whole-mount embryos collected from each of these lines were hybridized with a snail RNA probe, which labels developing haltere and wing discs in wild-type embryos Fig. 2. Enhancer piracy lines with dominant phenotypes. (A) A wild-type wing. (B) A line with typical strong extra wing vein phenotype. (C) A line with localized distal blister (arrow). (D) A line with severely thickened distal veins (between arrows). (E) A wild-type thorax viewed from above. Note the location of the two halteres posterior to the wings (arrows). (F-H) A line with various degrees of missing haltere and wing imaginal structures. (F) An individual with two missing halteres (arrows). (G) More strongly affected individuals with symmetric duplication of anterior wing structures. Arrow marks axis of symmetry. (H) An individual with the most severe shoulderless phenotype. Arrowhead marks dorsal midline. (I) A wild-type eye viewed from the side. (J) A line with rough eyes and ectopic orbital bristles (arrow). (K) A wild-type chorion. Note the location of dorsal appendages (arrow) near the dorsal midline and displaced approximately 20% of the length of the embryo in from the anterior tip. (L) A dominant female sterile line which lays eggs having dorsalized chorions. Note the extreme anterior and ventrolateral location of the dorsal appendages (arrow), consistent with a dramatic expansion of dorsal positional values at the expense of ventral most structures. This panel is a composite of two different focal planes so that the pattern of the eggshell can be seen as well as the location of the dorsal appendages. 2332 R. Noll and others Enhancer piracy and rhomboid function 2333 Fig. 3. Embryonic phenotype of enhancer piracy line missing haltere and wing disc structures. (A) Whole-mount in situ hybridization of a snail RNA digoxigenin-labeled probe to a wild-type 12hour embryo (viewed from the side with anterior to the left). The haltere (h) and wing (w) disc primordia are labeled at this stage (Alberga et al., 1991). (B) snail expression in the enhancer piracy line missing haltere and wing disc structures in the adult (same line shown in Figs 2F-H). The haltere disc is missing and the wing disc is reduced in size. (C) Two pairs of snail-expressing haltere and wing discs in a wild-type embryo viewed in horizontal section. (D) An embryo from the same enhancer piracy line shown in B; two haltere discs and one wing disc are missing (viewed in horizontal section). (Alberga et al., 1991; Fig. 3A,C). Mutant embryos frequently exhibit reduced numbers of sna-expressing haltere disc cells and often entirely lack sna expression in at least one haltere disc (Fig, 3B). sna expression was also affected in all or portions of wing discs (Fig. 3D). In extreme cases, sna expression in all haltere and wing disc primordia was eliminated (data not shown). Although rough eyes, as illustrated by the enhancer piracy line shown in Fig. 2J, have been observed following heat induction of HS-rho lines during the early third larval instar (Freeman et al., 1992), ectopic orbital bristles (arrow) have not been recovered in heat shock experiments. A rare and poorly penetrant phenotype observed in this screen was a distorted leg similar to the ‘malformed’ phenotype described for mutants in the broad locus (Gotwals and Fristrom, 1991). Finally, because ectopic rho expression during oogenesis dorsalizes follicle cells (Ruohola-Baker et al., 1993), we screened 113 lines for dominant female sterility and found one highly penetrant dominant female sterile line. Comparison of egg shells laid by wild-type females (Fig. 2K) to those deposited by the dominant female sterile line (Fig. 2L) revealed that the chorions are indeed dorsalized. Enhancer piracy alleles interact genetically with mutations in the EGF-R/RAS signaling pathway The basis for the various rho enhancer piracy phenotypes is not known. However, based on our previous analysis of misexpressing rho during wing vein development (Sturtevant et al., 1993), one explanation is that rho hyperactivates EGF-R signaling in inappropriate cells and consequently disrupts Table 2. Genetic interactions between enhancer piracy lines and mutants in components of the ventrolateral signaling pathway Test Mutant Genotype Egf-rElp + Ras1e1b + ±↓ 0 ±↓ 0 ↓ ±↓ ↑↑† ±↓ ↑ 0‡ 26 94/361 19 7/37 20 2/10 0 0/54 52 29/56 0 0/29 0.5 2/392 18.5§,12.5§ 35/191,10/80 0.4 1/248 26.5 102/384 0.2 1/462 25.1 120/479 46 (36) 39 (30) 15 (12) 70 (39) 20 (11) 11 (6) 52 (33) 38 (24) 11 (7) 57 (31) 32 (17) 11 (6) 28 (11) 38 (15) 35 (14) 78 (52) 19(13) 3 (2) N=78 N=56 N=64 N=54 N=40 N=67 + + Df Egf-r + Extra orbital bristles* all flies Rough eye* subtle Serrated wing margin (%) (no /total) Female sterility (% hatched) (no. hatched/total) Enhancer piracy phenotype Haltereless: 2 halteres: % (no.) 1 haltere: % (no.) 0 halteres: % (no.) Total no. of flies ± indicates weak interaction; ↓ indicates suppression; ↑ indicates enhancement; 0 indicates no modification of the phenotype. *Extra orbital and brisles and rough eye scored in same enhancer piracy line (see Fig. 2I,J). †Eye is typically reduced to about half the size observed in Egf-rElp alone. ‡Eye has unmodified Star phenotype. §Strong Egf-r alleles Egf-rIK35 (first entry) and Egf-r2L65 (second entry) were used in place of DfEgf-r. Gap1B2 + Starl(2)54 + 2334 R. Noll and others normal development. It is also possible that some of the dominant phenotypes observed result from interference with developmental decisions that normally do not involve rho or the EGF-R/RAS pathway. To distinguish between these possibilities, we crossed enhancer piracy lines with dominant phenotypes including extra orbital bristles, rough eyes, serrated wings, or female sterility with flies carrying mutations in components of the EGF-R signaling pathway and scored for changes in the magnitude of the dominant phenotypes in the trans-heterozygous progeny. Results from these crosses, summarized in Table 2, reveal that the various enhancer piracy phenotypes are strongly modified by mutations in the EGF-R/RAS pathway in a pattern that is consistent with rho acting together with Star to promote signaling through EGF-R in each of these cases. Thus, heterozygosity for loss of function mutations in Egf-r, Ras1, or Star tend to suppress the various rho enhancer piracy phenotypes, while the hyperactive Egf-rElp allele or a loss of function Gap1 allele enhances these phenotypes. As in the case of HS-rho lines having extra vein phenotypes (Sturtevant et al., 1993), Star is consistently the most potent suppressor of dominant rho phenotypes. As mentioned earlier, the dominant female sterile enhancer piracy line lays eggs with ventrally shifted dorsal appendages (Figs 2J, 4A,B). We asked whether the strong suppression of female sterility observed with Egf-r, Ras1, and Star (Table 2) was associated with a reduction of the dorsalized eggshell phenotype. The dorsalized eggshell phenotype was partially suppressed by removing one active copy of Egf-r (Fig. 4C,D) and eggs laid by mothers trans-heterozygous for either Ras1 (Fig. 4E,F) or for Star (Fig. 4G,H) typically had dorsal appendages in nearly wild-type locations (compare with Fig. 2K). The location of the dorsal appendages was not appreciably altered by heterozygous Gap1 or by Egf-rElp. Expression of the rho transgene in enhancer piracy lines correlates with localized phenotypes To verify our expectation that the localized phenotypes recovered in several rho enhancer piracy lines were due to spatially restricted expression of the rho transgene, we hybridized appropriate tissues with a probe specific for the 3′ transcribed trailer provided by the HS vector (Fig. 5). The pattern of rho transgene expression in wing discs of the line having entire sectors of the wing converted to vein (Fig. 2D) corresponds precisely to the affected regions of the wing (Fig. 5B,C; between arrows, but not between veins L3 and L4). Consistent with the localized distal wing blister phenotype of the line shown in Fig. 2C, ectopic expression of Fig. 4. Suppression of the dorsalized eggshell phenotype by Ras1 and Star mutations. Eggs were collected from mothers heterozygous for the dominant female sterile enhancer piracy insertion alone (A,B) or in combination with a heterozygous test mutation (C-F). Arrows indicate the anterior-posterior locations of the dorsal appendages relative to the anterior end of the eggs, which are marked by a stars. (A,B) The eggs shown in these panels are typical examples of dorsalized phenotypes observed in collections from this line. An example of more strongly dorsalized eggs present in the same collection is shown in Fig. 2J. (C,D) Eggs from female sterile mothers also heterozygous for Egf-r2L65. The dorsalized eggshell phenotype is partially suppressed. (E,F) Eggs from female sterile mothers also heterozygous for Ras1e1B. Note that the dorsal appendages are very close to the dorsal midline and at about 20% egg length as in wild type - see Fig. 2K for comparison. (G,H) Female sterile mothers heterozygous for Stare(2)54 also lay eggs with nearly wild-type appearance. Enhancer piracy and rhomboid function 2335 Fig. 5. Spatially restricted enhancer driven expression of the rho transgene in enhancer piracy lines with localized dominant phenotypes. (A) Expression of endogenous rho gene during the late third instar in wing vein primordia. The future wing margin is labeled M and the longitudinal vein primordia are numbered L1-L5. Arrows mark the primordia for the region between L2 and L5. (B) Expression of the rho transgene during the late third instar in the enhancer piracy line with entire intervein sectors converted to veins (Fig. 2D). Continuous vein sectors derive from the region between the arrows (i.e. between L2 and L5). There is a gap in transgene expression in the intervein sector between primordia for L3 and L4 which provides an explanation for the fact that ectopic veins are excluded from this region in the transformant line. (C) Expression of the rho transgene 7-8 hours after puparium formation (AP) in the same enhancer piracy line as that shown in B (see D for the normal rho expression pattern in vein primordia at this stage). (D) Expression of endogenous rho gene at 7-8 hours AP in future wing vein cells. (E) Expression of the rho transgene at 7-8 hours AP in the enhancer piracy line with a distal blister (Fig. 2C). (F) Lower magnification view of E including rho transgene expression in the developing wing (w) haltere (h) and leg (l). (G) Localized expression of the rho transgene in a strip of cells separating the eye (e) and antennal (a) primordia of the eye disc in the line with extra orbital bristles (Fig. 2J). By this stage of the orderly array of developing ommatidial preclusters is irregular, and presumably underlies the final adult rough eye phenotype. Ectopic expression of rho at earlier developmental stages in the eye disc, which is particularly intense in the morphogenetic furrow (data not shown), may be responsible for this phenotype. (H,I) Expression of the rho transgene in the haltereless line shown in Fig. 2F-H. The patches of cells in thoracic segments T2 and T3 are in the approximate locations where the wing and haltere disc cells develop. The consequences of transgene expression in similar clusters of cells in gnathal segments G2 and G3, in T1, and in abdominal (A) segments are unknown. cf, cephalic furrow. the rho transgene in this line is restricted to a distal section of the wing during the prepupal period (arrow in Fig. 5E). Other discs also exhibit localized expression of the transgene at this stage (Fig. 5F) including the distal tip of the haltere disc (arrowhead) and stripes in the leg disc (stars). Restriction of rho transgene expression to distal portions of both the wing and haltere discs may reflect their common evolutionary origin. The rho transgene in the transformant line shown in Fig. 2J (which has rough eyes and extra orbital bristles) is expressed in a strip of cells between the eye and antennal domains of the eye-antennal disc (Fig. 5G). The extra bristles observed in this transformant derive from this region of the 2336 R. Noll and others disc (star). Earlier during eye disc development, the transgene is expressed at high levels in the morphogenetic furrow (data not shown). This early expression is likely to underlie the final rough eye phenotype as heat induction experiments indicate that mystery cells can be converted into photoreceptor precursor cells by ectopic rho expression (Freeman et al., 1992). Finally, we examined expression of the rho transgene in embryos of the haltereless line shown in Fig. 2F-H. The transgene is expressed in a segmentally repeated pattern of small patches of cells at germband extension (Fig. 5H,I). Groups of cells in thoracic segments T2 and T3 are at the approximate locations from which wing and haltere discs subsequently form in wild-type embryos. In contrast, the endogenous rho gene is not expressed in wing or haltere disc primordia during embryogenesis. The lethality of the P-element insertion in the haltereless enhancer piracy line shown in Fig. 2 F-G is not allelic to sna, eliminating the remote possibility that embryos exhibiting a loss of sna expression are homozygous for an insertion into the sna gene itself. The isolation of a second independent viable haltereless enhancer piracy line also supports the view that the dominant loss of halteres results from ectopic rho expression. Thus, in several cases, the pattern of localized rho transgene expression in enhancer piracy lines correlates with the regions that fail to differentiate normally, suggesting that the diverse phenotypes recovered by this approach are indeed due to localized mis-expression of rho. DISCUSSION The mechanism by which ectopic rho expression leads to the variety of defects observed in the different enhancer piracy lines remains to be determined. However, mutations in the EGF-R/RAS signaling pathway modify several of these phenotypes, as would be expected if rho were functioning to hyperactivate EGF-R signaling in each of these cases. These results generalize previous findings (Rutledge et al., 1992; Sturtevant et al., 1993; Ruohola-Baker et al., 1993) in which rho was proposed to promote EGF-R signaling. The consistently strong suppression of dominant rho phenotypes by Star also implicates Star as another generally required component for facilitating EGF-R signaling. These dominant rho phenotypes, which are predictably modified by known mutations in the EGF-R signaling pathway, are suitable for identifying new loci interacting with rho (see below). Dominant alleles are powerful genetic tools that can be used for identifying additional genes functioning in a given pathway. For example, in the case of rho, dominant extra wing vein phenotypes are predictably modified in trans-heterozygous combinations with mutants in the EGF-R signaling pathway (Sturtevant et al., 1993). These interactions are opposite to interactions observed with loss of function rho alleles. As mentioned above, this array of genetic interactions strongly supports models in which rho functions to facilitate or amplify EGF-R signaling. Several of the rho enhancer piracy lines are currently being used to search for new second site suppressors of dominant rho phenotypes. Comparing the groups of interacting genes recovered in such screens based on suppression of different dominant rho phenotypes (e.g. extra wing veins versus dominant female sterility) should help dis- tinguish genes acting at only one developmental stage from those required in general for EGF-R signaling. A striking feature of this screen was that many of the dominant phenotypes recovered were not observed in a series of heat induction experiments (Sturtevant et al., 1993; Ruohola-Baker et al., 1993; J.W. O’Neill and E. Bier, unpublished data; M. Sturtevant and E. Bier, unpublished data). These relatively rare dominant enhancer piracy phenotypes might be difficult to recover by ubiquitous expression via heat shock induction because ubiquitous expression at particular stages is organismic lethal, or because the heat shock promoter cannot provide high enough levels of target gene expression to generate a phenotype. It is possible that some of the observed phenotypes could be due to dominant inactivation of a gene residing at the insertion site and not due to ectopic rho expression. While we cannot rule out this possibility for each individual case, it is highly unlikely that events of this kind contribute significantly to the data in Table 1. First, dominant phenotypes due to P-element insertion are exceedingly rare. In screens of several thousand enhancer trap lines, only a small handful of dominant visible phenotypes were recovered (Bier et al., 1989). Furthermore, in nearly every case in this enhancer piracy screen, we found several independent lines with a given dominant phenotype. Since P-element insertion into the ‘hottest’ target sites is on the order of 1/400 and are more typically in the range of 1/2,000 (Bier et al., 1989), it is very unlikely that recovery of multiple examples of a given dominant phenotype among 250 lines is due to independent insertions into the same locus. Two rare dominant alleles recovered in the enhancer piracy screen provide insights into the developmental function of rho that were not revealed from ubiquitous ectopic rho expression (Sturtevant et al., 1993; M. Sturtevant and E. Bier, unpublished data). The enhancer piracy line in which entire intervein sectors are converted to vein demonstrates that rho is sufficient to convert all intervein cells to the vein fate. A detailed analysis of phenotypes resulting from staged heat inductions of an inducible HS-rho line failed to reveal this potential since only cells near forming veins or cells in particular stereotyped intervein locations were highly susceptible to being transformed into a vein fate by ectopic rho expression (Sturtevant et al., 1993; M. Sturtevant and E. Bier, unpublished data). As the rho transgene is transiently expressed at high levels in sharply localized patterns corresponding to the primordia of affected wing sectors, this line underscores the value of enhancer piracy in determining the range of consequences resulting from tightly targeted mis-expression of rho. Thus, although other gene(s) function in parallel with rho to mediate the localized formation of veins (Sturtevant et al., 1993), optimal mis-expression of rho is sufficient to convert all intervein cells into veins. This suggests that when rho is expressed in such a pattern, gene(s) mediating parallel vein induction pathway(s) are either not required or are expressed at functional levels in intervein territories as well as in vein primordia. The other novel class of phenotypes recovered is missing halteres and or abnormal wing development (Fig. 2F-H). These phenotypes derive from an early developmental defect during embryogenesis since formation of haltere and wing disc primordia is abnormal. It is interesting in this regard that similar phenotypes have been observed in various combina- Enhancer piracy and rhomboid function 2337 tions of pointed alleles (Scholz et al., 1993). It is unclear whether these phenotypes result from loss or gain of pointed function. Embryos lacking pointed function have defects in the same structures requiring the activity of rho and other ventrolateral group genes (Mayer and Nüsslein-Volhard, 1988; Klämbt, 1993). Thus, regulated activity of the ventrolateral pathway may be required for the formation of wing and haltere discs. The fact that it is possible to recover enhancer piracy lines with localized wing venation defects and that the rho transgene is expressed in corresponding localized patterns in developing wing primordia suggests that enhancer piracy can be used like enhancer trapping to identify genes expressed in novel localized patterns. For example, in the case of wing development, it should be efficient to search for enhancer elements directing localized gene expression in wing discs using enhancer piracy by screening for lines with localized ectopic veins. This point is well illustrated by the pattern of rho transgene expression (Fig. 5B,C) in wing discs of the enhancer piracy shown in Fig. 2C. Further genetic analysis will be required to determine whether this enhancer piracy insertion is in or near a gene required for normal wing development. There is good evidence that enhancer piracy will be useful for generating dominant alleles of other genes of interest. In independent studies using the same hs-CaSpeR vector employed in this study, distinct position-dependent dominant phenotypes have been observed for constructs carrying diverse cDNAs for genes such as Suppressor of hairless Su(H) (Schweisguth and Posakony, 1994), Hairless (H), Bearded (Brd), and the HLH encoding genes, achaete (ac) and scute (sc) (J.W. Posakony, personal communication). In the case of HS-Su(H), dominant phenotypes behave genetically as would be expected for hypomorphic alleles (Schweisguth and Posakony, 1994). Other evidence, also suggests that similar strategies for mis-expressing genes of interest should be generally useful. For example, ectopic expression of an activated RAS gene leads to an excess wing vein phenotype very similar to that shown for rho in Fig. 2B (Brand and Perrimon, 1993). Patterned mis-expression of the ubiquitously expressed Serrate (Ser) gene also yields localized defects in a subset of cells driving high levels of a Ser transgene (Speicher et al., 1994). Finally, localized mis-expression of the homeobox transcription factor encoded by the Antennapedia gene resulting from a chromosomal rearrangement that brings this transcription unit under the control of a head specific promoter, generates a classic homeotic transformation of antennae to legs (Schneuwly et al., 1987). Investigators studying various other genes have also inadvertently recovered dominant alleles resulting from constitutive activation of cDNAs carried on heat-shock vectors, which in some cases have served as important genetic tools (Van Vactor et al., 1991). Thus, it is likely that the enhancer piracy method will be useful for obtaining dominant alleles of many other genes, especially those genes for which known phenotypes result from heat shock expression. In principle, enhancer piracy is applicable to a wide variety of situations in which regulated expression of the gene of interest is important for the development or survival of a dispensable region of the organism. It is worth noting that enhancer piracy, while similar in certain respects to the GAL4 mediated conditional expression of target genes (Brand and Perrimon, 1993), offers comple- mentary advantages for obtaining useful genetic tools. First, only a single genetic element is required for enhancer piracy, whereas the GAL4 system requires both a GAL4 producing element and a responding UAS-cDNA construct. Second, GAL4 activation is indirect and may not achieve the high levels of expression derived from direct activation of the target gene by powerful genomic enhancers. These intrinsic advantages and the relatively high frequency with which dominant phenotypes are recovered suggest that enhancer piracy will prove to be an effective general method for obtaining simple dominant alleles. The two component GAL4 system also offers important advantages as conditional ectopic expression of genes in known patterns permits analysis of ectopic expression leading to lethality, which cannot be studied using enhancer piracy. Dominant alleles arising from ectopic expression of a transgene are also of significant value for recovering large numbers of mutant alleles of that transgene, since reverting a dominant phenotype requires only an F1 screen. For genes such as rho, which do not share obvious primary sequence similarity with other genes of known function, the ability to generate large numbers of mutant alleles of the transgene provides an important tool for identifying critical functional domains of the encoded protein product. Preliminary data, using EMS as a mutagen, indicate that null revertants of a HS-rho dominant extra vein phenotype can be recovered at a frequency of once per 2,000-3,000 chromosomes (K. Howard and E. Bier, unpublished results). Since the mutagenized rho transgene can be distinguished from the endogenous rho gene by virtue of flanking P-element vector sequences, it is possible to characterize only the mutated copy of rho by using the polymerase chain reaction (PCR). Primers synthesized to unique flanking vector sequences can be used for PCR to amplify the entire rho coding region (only 1 kb long), which can then be directly sequenced to identify the mutant amino acid residue. This use of enhancer piracy should be applicable to many other genes as protein coding portions of cDNAs are frequently short enough to be amplified in their entire length (< 3 kb) or in two overlapping segments (< 6 kb). Since transposable elements have been isolated from a broad spectrum of organisms including mariner elements in diverse insects (Robertson, 1993), Tc elements in C. elegans and other nematodes (Abad et al., 1991), Ac/Ds elements in maize, tobacco, tomatoes, and Arabidopsis (Walbot, 1992; Grevelding et al., 1992; Bancroft and Dean, 1993), and the endogenous Tag1 element in Arabidopsis (Tsay et al., 1993), it is likely that the enhancer piracy approach could be generalized for isolating dominant phenotypes for genes of interest in these organisms. Given the increasing efficiency of generating other transgenic organisms such as mice (Korn et al., 1992), fish (Bayer and Campos-Ortega, 1992), and various plants (Walbot, 1992; Topping et al., 1991), similar strategies based on random genomic insertion of cDNA constructs driven by basal promoters may soon be feasible in these organisms as well. We thank Jason O’Neill for help with whole-mount in situ hybridization; Dan Lindsley, James W. Posakony, Bob Schmidt, Martin Yanofsky, Nigel Crawford, Hugo Bellen and Kathryn S. Burton for helpful discussions and critical comments on the manuscript; James W. Posakony for communicating unpublished results; and Kathryn S. Burton for assembling the figures. This work was supported by NIH 2338 R. Noll and others Grant no. RO1-NS29870-01, NSF Grant no. IBN-9318242 and Research Grant no. 5-FY92-1175 from the March of Dimes Birth Defects Foundation. E. B. was supported by funds from the McKnight Neuroscience Foundation, Sloan Foundation, and an ACS Junior Faculty Award. REFERENCES Abad, P., Quiles, C., Tares, S., Piotte, C., Castagnoe-Sereno, P., Abadon, M. and Dalmasso, A. (1991). Sequences homologous to Tc(s) transposable elements of Caenorhabitidis elegans are widely distributed in the phylum Nematoda. J. Mol. Evol. 33, 251-258. Alberga, A., Boulay, J.-L., Kempe, E., Dennefeld, C. and Haenlin, M. (1991). The snail gene required for mesoderm formation in Drosophila is expressed dynamically in derivatives of all three germ layers. Development 111, 983-992. Ashburner, M., Thompson, P., Roote, J., Lasko, P. F., Grau, Y., El Messal, M., Roth, S. and Simpson, P. (1990). The genetics of a small autosomal region of Drosophila melanogaster containing the structural gene for alcohol dehydrogenase. VII. Characterization of the region around the snail and cactus loci. Genetics 126, 679-694. Bancroft, I. and Dean, C. (1993). Transposition patter of the maize element Ds in Arabidopsis thaliana. Genetics 134, 1221-1229. Bang, A. G. and Posakony, J. W. (1992). The Drosophila gene Hairless encodes a novel basic protein that controls alternative cell fates in adult sensory organ development. Genes Dev. 6, 1752-1769. Bayer, T. A. and Campos-Ortega, J. (1992). A transgene containing lacZ is expressed in primary sensory neurons in zebrafish. Development 115, 421426. Bellen, H. J, O’Kane, C. J., Wilson, C., Grossniklaus, U., Pearson, R. K. and Gehring, W. J. (1989). P-element-mediated enhancer detection: a versatile method to study development in Drosophila. Genes Dev. 3, 12881300. Bier, E., Vaessin, H., Shepherd, S., Lee, K., McCall, K., Barbel, S., Ackerman, L., Carretto, R., Uemura, T., Grell, E., Jan, L. Y. and Jan, Y. N. (1989). Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes Dev. 3, 1273-1287. Bier, E., Jan, L. Y. and Jan, Y. N. (1990). rhomboid, a gene required for dorsoventral axis establishment and peripheral nervous system development in Drosophila melanogaster. Genes Dev. 4, 190-203. Brand, A. H. and Perrimon, N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415. Clifford, R. J. and Schüpbach, T. (1992). The torpedo (DER) receptor tyrosine kinase is required at multiple times during Drosophila embryogenesis. Development 115, 853-872. Freeman, M., Kimmel, B. E. and Rubin, G. M. (1992). Identifying targets of the rough homeobox gene of Drosophila: evidence that rhomboid functions in eye development. Development 116, 335-346. Gotwals, P. and Fristrom, J. W. (1991). Three neighboring genes interact with the Broad-complex and the Stubble-stubbleoid locus to affect imaginal disc morphogenesis in Drosophila. Genetics 127, 747-759. Grevelding, C., Becker, D., Kunze, R., von Menges, A., Fantes, V., Schell, J. and Masterson, R. (1992). High rates of Ac/Ds germinal transposition in Arabidopsis suitable for gene isolation by insertional mutatgenesis. Proc. Nat. Acad. Sci. 89, 6085-6089. Klämbt, C. (1993). The Drosophila gene pointed encodes two ETS-like proteins which are involved in the development of the midline glial cells. Development 117, 163-176. Korn, R., Schoor, M., Neuhaus, H., Henseling, U., Soininen, R., Zachgo, J. and Gossler, A. (1992). Enhancer trap integrations in mouse embryonic stem cells give rise to staining patterns in chimeric embryos with a high frequency and detect endogenous genes. Mech Dev. 39, 95-109. Lindsley, D. L. and Grell, E. H. (1968). Genetic variations in Drosophila melanogaster. Carnegie Institute of Washington, Washington, D. C. Lindsley, D. L. and Zimm, G. G. (1992). The Genome of Drosophila melanogaster. San Diego, CA: Academic Press Inc. Maniatis, T., Fritsch, E. F. and Sambrook, J. (1982). Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. Mayer, U. and Nüsslein-Volhard, C. (1988). A group of genes required for pattern formation in the ventral ectoderm of the Drosophila embryo. Genes Dev. 2, 1496-1511. O’Kane, C. and Gehring, W. (1987). Detection in situ of genomic regulatory elements in Drosophila. Proc. Nat. Acad. Sci. USA 84, 9123-9127. Raz, E. and Shilo. B. Z. (1992). Dissection of the faint little ball (FLB) phenotype – determination of the development of the Drosophila central nervous system by early interactions in the Ectoderm. Development 114, 113-123. Raz, E. and Shilo, B. Z. (1993). Establishment of ventral cell fates in the Drosophila ectoderm requires DER, the EGF receptor homolog. Genes Dev. 7, 1937-1948. Roberts, D. B. (1986). Basic Drosophila care and techniques. In Drosophila: A Practical Approach (ed. D. B. Roberts), pp. 1-38. Washington, D. C., IRL Press. Robertson, H. M. (1993). The mariner transposable element is widespread in insects. Nature 362, 241-245. Ruohola-Baker, H., Grell, E., Chou, T. B., Baker, D., Jan, L. Y. and Jan, Y. N. (1993). Spatially localized rhomboid is required for establishment of the dorsal-ventral axis in Drosophila oogenesis. Cell 73, 953-965. Rutledge, B., Zhang, K., Bier, E., Jan, Y. N. and Perrimon, N. (1992). The Drosophila spitz gene encodes a putative EGF-like growth factor involved in dorsal-ventral axis formation and neurogenesis. Genes Dev. 6, 1503-1517. Schneuwly, S., Klemenz, R. and Gehring, W. (1987). Molecular analysis of the dominant homeotic Antennapedia phenotype. EMBO J. 6, 201-206. Scholz, H., Deatrick, J., Klaes, A. and Klampt, C. (1993). Genetic dissection of pointed, a Drosophila gene encoding two ETS-related proteins. Genetics 135, 455-468. Schweisguth, F. and Posakony, J. W. (1994). Antagonistic activities of Suppressor of Hairless and hairless control alternative cell fates in the Drosophila adult epidermis. Development 120, 1433-1441. Speicher, S. A., Thomas, U., Hinz, U. and Knust, E. (1994). The Serrate locus of Drosophila and its role in morphogenesis of the wing imaginal discs: control of cell proliferation. Development 120, 535-544. Sturtevant, M. A., Roark, M. and Bier, E. (1993). The Drosophila rhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGF-R signaling pathway. Genes Dev. 7, 961-973. Tautz, D. and Pfeiffle, C. (1989). A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila reveals translational control of the segmentation gene hunchback. Chromosoma 98, 81-85. Topping, J. F., Wei, W. and Lindsey, K. (1991). Functional tagging of regulatory elements in the plant genome. Development 112, 1009-1019. Tsay, Y.-F., Frank, M. J., Page, T., Dean, C. and Crawford, N. M. (1993). Identification of a mobile endogenous transposon in Arabidopsis thaliana. Science 260, 342-344. Van Vactor, D. L., Cagan, R. L., Kramer, H. and Zipursky, S. L. (1991). Induction in the developing compound eye of Drosophila: multiple mechanisms restrict R7 induction to a single retinal precursor cell. Cell 67, 1145-1155. Walbot, V. (1992). Strategies for mutagenesis and gene cloning using transposon tagging and T-DNA insertional mutatgenesis. Ann. Rev. Plant Phys. Plant Mol. Biol. 43, 49-89. Wilson, C., Pearson, R. K., Bellen, H. J., O’Kane, C. J., Grossniklaus, U. and Gehring, W. (1989). P-element-mediated enhancer detection: an efficient method for isolating and characterizing developmentally regulated genes in Drosophila. Genes Dev. 3, 1301-1313. (Accepted 17 May 1994)