* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download מצגת של PowerPoint
Epigenetics of neurodegenerative diseases wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Public health genomics wikipedia , lookup
Behavioural genetics wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Biology and sexual orientation wikipedia , lookup
History of genetic engineering wikipedia , lookup
X-inactivation wikipedia , lookup
Dominance (genetics) wikipedia , lookup
Genome (book) wikipedia , lookup
Ridge (biology) wikipedia , lookup
Minimal genome wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Genome evolution wikipedia , lookup
Pathogenomics wikipedia , lookup
Gene expression programming wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
Microevolution wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Gene expression profiling wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Designer baby wikipedia , lookup
Epigenetics of human development wikipedia , lookup
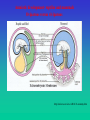
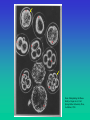
Genetic Imprinting No parthenogenesis in mammals Genomic imprinting and the strange case of the insulin-like growth factor II receptor. Haig D, Graham C. Cell. 64:1045-6 (1991) Maternal-Paternal Conflict Theory The sins of the fathers and mothers: genomic imprinting in mammalian development. Tilghman SM. Cell;96:185-93 (1999). A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. DeChiara TM, Efstratiadis A, Robertson EJ. KO Nature. 1990, 345,78-80. Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Lau MM… Stewart CL. Genes Dev. 1994 ;8, 2953-63. . Rescue of the T-associated maternal effect in mice carrying null mutations in Igf-2 and Igf2r, two reciprocally imprinted genes. Filson AJ, Louvi A, Efstratiadis A, Robertson EJ Development. 1993, 118 :731-6. I KO I I KO I KO Holmes: “When you have eliminated the impossible, whatever remains, however improbable, must be the truth” “Perhaps,” said Watson, skeptically, “You may have convinced me as to the motive, but you are yet to explain how it is done.” Tilghman SM. Cell 1999 ;96:185-93 A maternal-offspring coadaptation theory for the evolution of genomic imprinting. Wolf JB, Hager R. PLoS Biol. 2006 Nov;4(12):e380. Imprinted genes are expressed either from the maternally or paternally inherited copy only, and they play a key role in regulating complex biological processes, including offspring development and mother-offspring interactions. There are several competing theories attempting to explain the evolutionary origin of this monoallelic pattern of gene expression, but a prevailing view has emerged that holds that genomic imprinting is a consequence of conflict between maternal and paternal gene copies over maternal investment. However, many imprinting patterns and the apparent overabundance of maternally expressed genes remain unexplained and may be incompatible with current theory. Here we demonstrate that sole expression of maternal gene copies is favored by natural selection because it increases the adaptive integration of offspring and maternal genomes, leading to higher offspring fitness. This novel coadaptation theory for the evolution of genomic imprinting is consistent with results of recent studies on epigenetic effects, and it provides a testable hypothesis for the origin of previously unexplained major imprinting patterns across different taxa. In conjunction with existing hypotheses, our results suggest that imprinting may have evolved due to different selective pressures at different loci. Distinct physiological and behavioural functions for parental alleles of imprinted Grb10. Garfield AS…Ward A. Nature. 469(7331):534-8 (2011) Imprinted genes, defined by their preferential expression of a single parental allele, represent a subset of the mammalian genome and often have key roles in embryonic development, but also postnatal functions including energy homeostasis and behaviour. When the two parental alleles are unequally represented within a social group (when there is sex bias in dispersal and/or variance in reproductive success), imprinted genes may evolve to modulate social behaviour, although so far no such instance is known. Predominantly expressed from the maternal allele during embryogenesis, Grb10 encodes an intracellular adaptor protein that can interact with several receptor tyrosine kinases and downstream signalling molecules. Here we demonstrate that within the brain Grb10 is expressed from the paternal allele from fetal life into adulthood and that ablation of this expression engenders increased social dominance specifically among other aspects of social behaviour, a finding supported by the observed increase in allogrooming by paternal Grb10-deficient animals. Grb10 is, therefore, the first example of an imprinted gene that regulates social behaviour. It is also currently alone in exhibiting imprinted expression from each of the parental alleles in a tissue-specific manner, as loss of the peripherally expressed maternal allele leads to significant fetal and placental overgrowth. Thus Grb10 is, so far, a unique imprinted gene, able to influence distinct physiological processes, fetal growth and adult behaviour, owing to actions of the two parental alleles in different tissues. High-frequency generation of viable mice from engineered bi-maternal embryos Manabu Kawahara….& Tomohiro Kono. Nat Biotechnol. 25:1045-50 (2007) Mammalian development to adulthood typically requires both maternal and paternal genomes, because genomic imprinting places stringent limitations on mammalian development, strictly precluding parthenogenesis. Here we report the generation of bi-maternal embryos that develop at a high success rate equivalent to the rate obtained with in vitro fertilization of normal embryos. These bi-maternal mice developed into viable and fertile female adults. The bi-maternal embryos, distinct from parthenogenetic or gynogenetic conceptuses, were produced by the construction of oocytes from fully grown oocytes and nongrowing oocytes that contain double deletions in the H19 differentially methylated region (DMR) and the Dlk1-Dio3 intergenic germline–derived DMR. The results provide conclusive evidence that imprinted genes regulated by these two paternally methylated imprinting-control regions are the only paternal barrier that prevents the normal development of bi-maternal mouse fetuses to term. Early mammalian development Amniotes Biology (Kampbell & reece) Amniotic development- reptiles and mammals Oviparous versus viviparous http://universe-review.ca/R10-33-anatomy.htm Monozygotic twins Dizygotic twins (identical twins) (fraternal or non-identical twins) < 5 days 5-9 days > 9 days Blood group chimerism in human multiple births is not rare. van Dijk BA, Boomsma DI, de Man AJ. Am J Med Genet. 61:264-8. (1996). Twin blood group chimerism seems to be very rare in humans. The 30-40 previously reported cases usually were found by mere coincidence during routine blood grouping in hospitals or blood banks. Usually in these cases frank blood group mixtures of, for example, 50/50%, 25/75%, or 5/95% at most were seen. Smaller percentages are very difficult to notice during routine work-up. Using a sensitive fluorescence technique (sensitivity > 0.01%) we detected blood group chimerism in 32/415 (8%) twin pairs and 12/57 (21%) triplet pairs, respectively, which is a higher incidence than reported previously. Do monochorionic dizygotic twins increase after pregnancy by assisted reproductive technology? Miura K, Niikawa N. J Hum Genet.;50:1-6 (2005). העץ הפילוגנטי המשוער של היונקים Marsupialsיונקי הכיס Eutherians יונקי השליה יונקי ביב Monotremata Monotreme קיפודן ברווזן "Monotremes oviparous, ovum meroblastic" (Caldwell, September 2, 1884) Biology (Solomon, Berg & Martin) Life Marsupials- יונקי הכיס Koala Tasmanian wolf/tiger Biology (Solomon, Berg & Martin) 7 1 2 8 3 4 5 6 9 From: Manipulating the Mouse Embryo. Hogan et al., Cold Spring Harbor Laboratory Press, 2nd Edition, 1994. The first developmental decision: ICM or trophoblast The first developmental decision: ICM or trophoblast Dev Biol 9th Ed. (2010) The first developmental decision: ICM or trophoblast Trophoblast ICM Dev Biol 9th Ed. (2010) From: Manipulating the Mouse Embryo. Hogan et al., Cold Spring Harbor Laboratory Press, 2nd Edition, 1994. Fazleabas and Kim (2003). What Makes an Embryo Stick ? Science 299:355-6. ליאונרדו דה וינצ'י עובר ברחם ~1510