* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch 4_ Osmosis and Diffusion.pptx
Survey
Document related concepts
Model lipid bilayer wikipedia , lookup
Action potential wikipedia , lookup
Theories of general anaesthetic action wikipedia , lookup
P-type ATPase wikipedia , lookup
Lipid bilayer wikipedia , lookup
Cytokinesis wikipedia , lookup
SNARE (protein) wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Signal transduction wikipedia , lookup
Mechanosensitive channels wikipedia , lookup
Magnesium transporter wikipedia , lookup
Membrane potential wikipedia , lookup
List of types of proteins wikipedia , lookup
Transcript
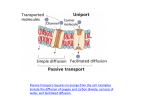
1/5/15 Chapter 4: Transport of Solutes and Water Transport? Solute? • Transport: movements through cell membranes or epithelia • Solutes: dissolved material being transported Transpor?ng solutes is cri?cal • Transport mechanisms are diverse because of high chemical diversity of solutes 1. Ions across internal cell membrane 2. Ions exchange from external environment (gill membranes) 3. Sugars and amino acids across intes?nal epithelia Two main transport mechanisms 1. Passive transport: carry material towards equilibrium 2. Ac?ve transport: capable of carrying solutes away from equilibrium Transpor?ng solutes is cri?cal • Transport mechanisms are diverse because of high chemical diversity of solutes 1. Diffusion 1. Passive 2. Ac?ve 2. Osmosis Two main transport mechanisms 1. Passive transport: carry material towards equilibrium – Simple diffusion – Facilitated diffusion Equilibrium: state that requires minimal amount of work to maintain 1 1/5/15 Passive transport: Simple Diffusion • WHAT? – Movement from areas of high concentra?on to low Rate of simple diffusion affected by: 1. Concentra?on gradient 2. Electrical gradient 3. Cell Membrane structure • WHY?? – Molecules in constant mo?on – Sta?s?cally more likely to move to lower concentra?ons 1. Concentra?on gradient 1. Magnitude of concentra?on gradient – How much concentra?on changes over given distance 2. Concentra?on of that par?cular solute 3. Membrane permeability to that par?cular solute 4. Temperature 2. Electrical gradient • Mo?on of charged ions depends on forces of aWrac?on/repulsion – Polar heads can maintain charge, aWrac?ng opposing charged ions Concentra?on gradient expressed as separate value for each solute! 3. Cell membrane structure • Hydrophobic interior of membrane – Speed determined by hydrophobic/philic proper?es of solute 3. Cell membrane structure 1. Lipid solutes are hydrophobic – Dissolve easily across membrane 2 1/5/15 3. Cell membrane structure 2. Hydrophilic inorganic ions à low lipid solubility à low membrane permeability Passive transport • Mediated by gated ion channels – Proteins that span the membrane and allow passage of inorganic ions into cell – Move through membrane via ion channels 4 types of gated ion channels 1. Voltage-‐gated 2. Stretch-‐gated 1. Voltage-‐gated channels • Open in response to voltage differen?al across membrane – Nerve impulses 3. Phosphoryla?on-‐gated 4. Ligand-‐gated 2. Stretch (tension)-‐gated channels 3. Phosphoryla?on-‐gated channels • Response to stretching or pulling forces that alter tension on membrane • Open when channel protein is phosphorylated – Mechanoreceptors in whiskers – Ac?va?on regulated by second messengers (phosphorous group) 3 1/5/15 4. Ligand-‐gated channel • Protein acts as receptor AND channel – Allow passage of selected ions only Permeability • Ease with which solute moves through cell membrane – Selec?vely permeable membranes only allow some ions through Electrochemical gradient • Concentra?on and electrical gradients can complement one another Two main transport mechanisms 1. Passive transport: carry material towards equilibrium – Simple Diffusion – Facilitated Diffusion Electrochemical gradient • Concentra?on and electrical gradients can complement one another…or oppose Facilitated diffusion 1. Always occurs in direc?on of electrochemical gradient 2. Facilitated diffusion is faster than simple diffusion 3. Does not require energy (like simple diffusion) Solutes bind to transporter proteins 4 1/5/15 Facilitated diffusion 1. Two main transport mechanisms Passive transport 2. Ac=ve transport: capable – Simple Diffusion – Facilitated Diffusion of carrying solutes away from equilibrium – Requires energy Ac?ve transport proper?es • Energy (ATP) supplied by catabolism of food • Transport of ions, amino acids and sugars across cell membranes Primary ac?ve transport: the proton pump – H2O and O2 ALWAYS move by PASSIVE transport • Movement of charged ions may or may not create voltage gradient – Electoroneutral vs. electrogenic VIDEO • Energy for movement of ions comes from ATP Secondary ac?ve transport: Transport of glucose • Energy iniAally supplied by voltage gradient – EXAMPLE: Glucose into intes?nes Transpor?ng solutes is cri?cal • Transport mechanisms are diverse because of high chemical diversity of solutes 1. Diffusion 1. Passive 2. Ac?ve 2. Osmosis 1. What is it? 2. How does it work? 5 1/5/15 Osmosis: What? • Passive transport of water across any membrane Osmosis: What? • Movement always towards higher osmo=c pressure – “diffusion of water” – Ac?ve water transport doesn’t occur – Faster than simple diffusion – Always towards equilibrium state – Measured as osmolarity units – Greater osmolarity= more dissolved units Fewer water molecules in blood plasma Water movement and osmo?c pressure • Isosmo=c: same osmo?c pressure • Hyposmo=c: a solu?on with lower osmo?c pressure, rela?ve to another solu?on • Hyperosmo=c: a solu?on with higher osmo?c pressure, rela?ve to another • Which direc?on would you expect solutes to move? QUIZ TIME! Fewer water molecules in blood plasma Osmosis: How? 1. Dissolve through membrane via diffusion 2. Channel-‐mediated transport – Channel proteins (e.g. aquaporins) – Faster than diffusion – More water channel proteins à greater membrane permeability BRIEFLY compare and contrast the following sets of terms: 1. Passive and ac?ve transport 2. Cell membranes and epithelia 3. Transmembrane and integral proteins 4. Nitrogen and energy metabolism 6