* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CITRIC ACID CYCLE
Catalytic triad wikipedia , lookup
Photosynthesis wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Butyric acid wikipedia , lookup
Electron transport chain wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Metalloprotein wikipedia , lookup
Mitochondrion wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Biosynthesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Biochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Amino acid synthesis wikipedia , lookup
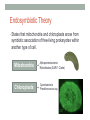
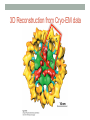
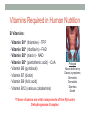
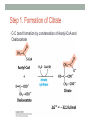
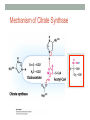
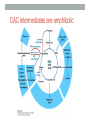
“Madala Energy”, 2012, oil on canvas, by Sonia Falcone CITRIC ACID CYCLE Lecture by Omar Delannoy April 14, 2014 What is energy? • Two main forms: Kinetic Potential Wikipedia.org Metabolism in living beings Eukaryotes VS. Prokaryotes Evolution of Aerobic Organisms • As early Earth was anoxic, all living cells were anaerobic and likely chemolithotrophic (autotrophic). • obtained carbon from CO2 • obtained energy from H2; likely generated by H2S reacting with H2S or UV light. • But then, • ~ 2.7 billion years ago, cyanobacterial lineages emerged with a photosystem that could use H2O instead of H2S, generating O2. Endosymbiotic Theory • States that mitochondria and chloroplasts arose from symbiotic association of free-living prokaryotes within another type of cell. Mitochondria Alphaproteobacteria Rickettsiales (SAR11 Clade) Chloroplasts Cyanobacteria Prochlorococcus sp. Cellular Respiration • Cells consume oxygen (O2) and produce carbon dioxide (CO2). • In most eukaryotes, these processes occur in the mitochondrion, while in aerobic prokaryotes, occur in the cytosol. • Do all eukaryotic cells have mitochondria? Giardia sp. • Genus of anaerobic protozoan parasites. • They lack mitochondria and Golgi apparatus. • These organisms are capable of colonizing the intestine of several vertebrates causing a condition name Giardiasis. Mitochondria • The major role of mitochondria is to produce energy through cellular respiration, but it has other functions as well. • It is involve in: • Signaling pathways • Cellular differentiation processes • Apoptosis • Regulation of cell cycle and cell growth Three Stages of Cellular Respiration Stage 1: Acetyl-CoA production • Generates some: • ATP • NADH • FADH2 Three Stages of Cellular Respiration Stage 2: Acetyl-CoA oxidation • Generates more: • NADH • FADH2 • and one GTP (or ATP) Three Stages of Cellular Respiration Stage 3: Oxidative Phosphorylation • Generates a lot of ATP Citric Acid Cycle Discovery • Hans A. Krebs and Fritz A. Lipmann shared the 1953 Nobel Prize in Physiology or Medicine for the discovery of the citric acid cycle and the co-enzyme A. Hans A. Krebs Fritz A. Lipmann Overview of the Citric Acid Cycle Production of Acetyl-CoA • Net Reaction: – Oxidative decarboxylation of pyruvate – First carbons of glucose to be fully oxidized • Catalyzed by the pyruvate dehydrogenase complex – Requires 5 coenzymes – TPP, lipoyllysine, and FAD are prosthetic groups – NAD+ and CoA-SH are co-substrates Structure of Coenzyme A • Coenzymes are not a permanent part of the enzymes’ structure. – They associate, fulfill a function, and dissociate • The function of CoA is to accept and carry acetyl groups Structure of Lipoyllysine (lipoate) • Prosthetic groups are strongly bound to the protein – The lipoic acid is covalently linked to the enzyme via a lysine residue. Pyruvate Dehydrogenase Complex (PDC) • PDC is a large (up to 10 MDa) multienzyme complex - pyruvate dehydrogenase (E1) - dihydrolipoyl transacetylase (E2) - dihydrolipoyl dehydrogenase (E3) • Advantages of multienzyme complexes: ‒ short distance between catalytic sites allows channeling of substrates from one catalytic site to another ‒ substrate channeling minimizes side reactions ‒ regulation of activity of one subunit affects the entire complex Cryoelectronmicroscopy of PDC • Samples are in near-native frozen hydrated state • Low temperature protects biological specimens against radiation damage • Electrons have smaller de Broglie wavelength and produce much higher resolution images than light 3D Reconstruction from Cryo-EM data Overall Reaction of PDC E2 E1 E3 Oxidative Decarboxylation of Pyruvate • Enzyme 1 • Step 1: Decarboxylation of pyruvate to an aldehyde • Step 2: Oxidation of aldehyde to a carboxylic acid ‒ Electrons reduce lipoamide and form a thioester • Enzyme 2 • Step 3: Formation of acetyl-CoA (product 1) • Enzyme 3 • Step 4: Reoxidation of the lipoamide cofactor • Step 5: Regeneration of the oxidized FAD cofactor ‒ Forming NADH (product 2) Vitamins Required in Human Nutrition B Vitamins: • Vitamin B1* (thiamine) - TPP • Vitamin B2* (riboflavin) - FAD • Vitamin B3* (niacin) - NAD • Vitamin B5* (pantothenic acid) - CoA • Vitamin B6 (pyridoxal) • Vitamin B7 (biotin) • Vitamin B9 (folic acid) • Vitamin B12 (various cobalamins) Pellagra Niacin deficiency Classic symptoms: Dementia Dermatitis Diarrhea Death *These vitamins are vital components of the Pyruvate Dehydrogenase Complex Overview of the Citric Acid Cycle Events in the Citric Acid Cycle • Step 1: C-C bond formation to make citrate • Step 2: Isomerization via dehydration/rehydration • Steps 3–4: Oxidative decarboxylations to give 2 NADH • Step 5: Substrate-level phosphorylation to give GTP • Step 6: Dehydrogenation to give reduced FADH2 • Step 7: Hydration • Step 8: Dehydrogenation to give NADH Step 1. Formation of Citrate • C-C bond formation by condensation of Acetyl-CoA and Oxaloacetate Enzyme: Citrate Synthase • Condensation of acetyl-CoA and oxaloacetate • The only reaction with C-C bond formation • Uses Acid/Base Catalysis • Carbonyl of oxaloacetate is a good electrophile • Methyl of acetyl-CoA is not a good nucleophile… • …unless activated by deprotonation • Rate-limiting step of CAC • Activity largely depends on [oxaloacetate] • Highly thermodynamically favorable/irreversible • Regulated by substrate availability and product inhibition Induced Fit in the Citrate Synthase • Conformational change occurs upon binding oxaloacetate • Avoids unnecessary hydrolysis of thioester in acetyl-CoA a) Open conformation: Free enzyme does not have a binding site for acetylCoA b) Closed conformation: Binding of OAA creates binding for acetyl-CoA Reactive carbanion is protected Induced Fit in the Citrate Synthase Mechanism of Citrate Synthase Step 2. Formation of Isocitrate by Dehydration/Rehydration Enzyme: Aconitase • Elimination of H2O from citrate gives a cis C=C bond • Lyase • Citrate, a tertiary alcohol, is a poor substrate for oxidation • Isocitrate, a secondary alcohol, is a good substrate for oxidation • Addition of H2O to cis-aconitate is stereospecific • Thermodynamically unfavorable/reversible • Product concentration kept low to pull forward Iron-Sulfur Center in Aconitase • Water removal from citrate and subsequent addition to cis-aconitate are catalyzed by the iron-sulfur center: sensitive to oxidative stress. Aconitase is stereospecific • Only R-isocitrate is produced by aconitase • Distinguished by three-point attachment to the active site Step 3. Oxidation of Isocitrate to form αketoglutarate and CO2 Oxidative Decarboxylation Enzyme: Isocitrate Dehydrogenase • Oxidative decarboxylation • Lose a carbon as CO2 • Generate NADH • Oxidation of the alcohol to a ketone • Transfers a hydride to NAD • Cytosolic isozyme uses NADP+ as a cofactor • Highly thermodynamically favorable/irreversible • Regulated by product inhibition and ATP Mechanisms of Isocitrate Dehydrogenase • 1) Metal Ion Catalysis (Oxidation) Mechanisms of Isocitrate Dehydrogenase • 2) Metal Ion Catalysis (Decarboxylation) • Carbon lost as CO2 did NOT come from acetyl-CoA. Mechanisms of Isocitrate Dehydrogenase • 3) Rearrangement and Product Release Step 4. Oxidation of α-ketoglutarate to Succinyl-CoA and CO2 Final Oxidative Decarboxylation Enzyme: α-Ketoglutarate Dehydrogenase • Last oxidative decarboxylation • Net full oxidation of all carbons of glucose • After two turns of the cycle • Carbons not directly from glucose because carbons lost came from oxaloacetate • Succinyl-CoA is another higher-energy thioester bond • Highly thermodynamically favorable/irreversible • Regulated by product inhibition Origin of C-atoms of CO2 • Both CO2 carbon atoms derived from oxaloacetate α-Ketoglutarate Dehydrogenase • Complex similar to pyruvate dehydrogenase • Same coenzymes, identical mechanisms • Active sites different to accommodate different-sized substrates Step 5. Conversion of Succinyl-CoA to Succinate • Generation of GTP through Thioester Enzyme: Succinyl-CoA Synthetase • Substrate level phosphorylation • Energy of thioester allows for incorporation of inorganic phosphate • Goes through a phospho-enzyme intermediate • Produces GTP, which can be converted to ATP • Slightly thermodynamically favorable/reversible • Product concentration kept low to pull forward Mechanism of Succinyl-CoA Synthetase Enzyme phosphorylation in His residue GTP to ATP conversion: Mg2+ GTP + ADP ΔG = 0 kJ/mol GDP + ATP Step 6. Oxidation of Succinate to Fumarate • Oxidation of an Alkane to Alkene Inhibition by malonate (competitive inhibitor of the dehydrogenase) – Blocks Krebs cycle. Enzyme: Succinate Dehydrogenase • Bound to mitochondrial inner membrane • Part of Complex II in the electron-transport chain • Reduction of the alkane to alkene requires FADH2 • Reduction potential of NAD is too low • FAD is covalently bound, unusual • Near equilibrium/reversible • Product concentration kept low to pull forward Step 7. Hydration of Fumarate to Malate • Hydration Across a Double Bond Enzyme: Fumarase • Stereospecific • Addition of water is always trans and forms L-malate • OH- adds to fumarate… then H+ adds to the carbanion • Cannot distinguish between inner carbons, so either can gain –OH • Slightly thermodynamically favorable/reversible • Product concentration kept low to pull reaction forward Stereospecificity of Fumarase Step 8. Oxidation of Malate to Oxaloacetate • Oxidation of Alcohol to a Ketone Enzyme: Malate Dehydrogenase • Final step of the cycle • Regenerates oxaloacetate for citrate synthase • Highly thermodynamically UNfavorable/reversible • Oxaloacetate concentration kept VERY low by citrate synthase • Pulls the reaction forward One Turn of the Citric Acid Cycle Net Result of the Citric Acid Cycle Acetyl-CoA + 3NAD+ + FAD + GDP + Pi + 2 H2O 2CO2 + 3NADH + FADH2 + GTP + CoA + 3H+ • Net oxidation of two carbons to CO2 • Equivalent to two carbons of acetyl-CoA • but NOT the exact same carbons • Energy captured by electron transfer to NADH and FADH2 • Generates 1 GTP, which can be converted to ATP • Completion of cycle Direct and Indirect ATP Yield CAC intermediates are amphibolic Anaplerotic Reactions • Intermediates in the citric acid cycle can be used in biosynthetic pathways (removed from cycle) • Must replenish the intermediates in order for the cycle and central metabolic pathway to continue • 4-carbon intermediates are formed by carboxylation of 3carbon precursors Regulation of the Citric Acid Cycle Regulation of the Citric Acid Cycle • Regulated at highly thermodynamically favorable and irreversible steps • PDH, citrate synthase, IDH, and KDH • General regulatory mechanism • Activated by substrate availability • Inhibited by product accumulation • Overall products of the pathway are NADH and ATP • Affect all regulated enzymes in the cycle • Inhibitors: NADH and ATP • Activators: NAD+ and AMP Regulation of Pyruvate Dehydrogenase • Also regulated by reversible phosphorylation of E1 • Phosphorylation: inactive • Dephosphorylation: active • PDH kinase and PDH phosphorylase are part of mammalian PDH complex • Kinase is activated by ATP • High ATP phosphorylated PDH less acetyl-CoA • Low ATP kinase is less active and phosphorylase removes phosphate from PDH more acetyl-CoA Additional Regulatory Mechanisms • Citrate synthase is also inhibited by succinyl-CoA • α-ketoglutarate is an important branch point for amino acid metabolism • Succinyl-CoA communicates flow at this branch point to the start of the cycle • Regulation of isocitrate dehydrogenase controls citrate levels • Aconitase is reversible • Inhibition of IDH leads to accumulation of isocitrate and reverses acconitase • Accumulated citrate leaves mitochondria and inhibits phosphofructokinase in glycolysis