* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download B4 Diffusion and osmosis
Signal transduction wikipedia , lookup
Cell growth wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell membrane wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
Endomembrane system wikipedia , lookup
Cytokinesis wikipedia , lookup
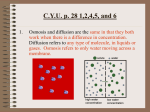
Diffusion and Osmosis – Revision Pack (B4) Diffusion: Diffusion is the movement of particles in a liquid or gas from an area of high concentration to an area of low concentration. It happens because of the random movement of individual particles. Diffusion explains how molecules like carbon dioxide, water and oxygen can get into and out of cells via the cell membrane. For example, if a plant is using up carbon dioxide then the concentration is low, so carbon dioxide will enter via diffusion. Leaves are adapted to increase the rate of diffusion of CO 2 and O 2 by: - Having a large surface area Having stomata (pores) which are spaced out Having gaps between spongy mesophyll cells The rate of diffusion can be increases by: - Having a short distance for molecules to travel Having a greater surface area for the molecules to diffuse into or out of Having a steeper concentration gradient (see below) In this instance, the concentration goes from an area of higher concentration, to an area of lower concentration. To increase the steepness of this gradient, the difference between the higher concentration and lower concentration must be greater. Osmosis: Osmosis is a type of diffusion. It needs a semi-permeable membrane that allows small molecules like water through, but doesn’t permit larger molecules like sugar through. Osmosis is the movement of water across a semi-permeable membrane from an area of high water concentration (dilute) to an area of low concentration (concentrated). Osmosis happens because of the random movement of water molecules, which are not restricted (like sugar is) by a semi-permeable membrane. The movement of water molecules will be from an area where there is lots to an area where there is few. If we know the concentration of water inside and outside a cell, we can predict the movement of those water molecules. Diffusion and Osmosis – Revision Pack (B4) Water in Cells: wall) When lots of water enters a plant cell, the pressure pushing on the cell wall is high. This is a high turgor pressure, which supports the cell – stopping it, and the whole plant, from collapsing. When too much water leaves a plant then it has a low turgor pressure and the plant will become limp. A plant cell that is full of water is called turgid. When a plant cell loses water we call the cell flaccid. NOTE – when a cell is plasmolysed, the cytoplasm is pulled away from the wall. Animal cells react in the same way as plant cells do towards water loss and water intake. When too much water is lost, animal cells will shrink and collapse. When too much water enters an animal cell, the cell will also swell up. Unlike plant cells, animal cells (like red blood cells) do not have a supporting cell wall. This means that when too much water enters an animal cell, they swell up and burst (image 3) – this is called lysis. When too much water leaves an animal cell, it shrinks into a scalloped shape (image 1) – this is called crenation. A normal animal cell is actually more flaccid than it is turgid (image 2). Diffusion and Osmosis – Revision Pack (B4) Past Papers: PPQ(1): PPQ(2): Diffusion and Osmosis – Revision Pack (B4) Diffusion and Osmosis – Revision Pack (B4) PPQ(3): Diffusion and Osmosis – Revision Pack (B4) Diffusion and Osmosis – Revision Pack (B4) Mark Schemes: PPQ(1): PPQ(2): PPQ(3): PPQ(4): Diffusion and Osmosis – Revision Pack (B4)