* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
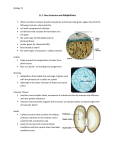
Download Shininger, AnnRev. Plant Physiol. 1979 30:313-37
Cytokinesis wikipedia , lookup
Cell growth wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell culture wikipedia , lookup
Programmed cell death wikipedia , lookup
Extracellular matrix wikipedia , lookup
List of types of proteins wikipedia , lookup
Tissue engineering wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cellular differentiation wikipedia , lookup
Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. AnnRev. Plant Physio£1979. 30:313-37 Copyright© 1979by AnnualReviewsInc. All rights reserved THE CONTROL OF VASCULAR 1DEVELOPMENT ~7674 Terry L. Shininger Department of Biology, Universityof Utah, Salt LakeCity, Utah84112 CONTENTS INTRODUCTION .......................................................................................................... PROCAMBIUM: DEVELOPMENT IN THESHOOT .............................................. THE CONTROL OF PRIMARY VASCULAR DIFFERENTIATION IN THE SHOOT .......................................................................................................... PROCAMBIUM: DEVELOPMENT IN THEROOT ................................................ CYTOLOGICAL AND BIOCHEMICAL ASPECTS OF VASCULAR DIFFERENTIATION .................................................................................. Cytological Aspects. ..................................................................................................... Biochemical Aspects .................................................................................................... THE CONTROLOF NON-PRIMARYVASCULARDIFFERENTIATION .......... Differentiation fromtheCambium ............................................................................ Experimentally Induced VascularDevelopment ........................................................ Wound-induced vascularization .................................................................................. Vascularization incultured tissue.............................................................................. CONCLUSIONS .............................................................................................................. 313 314 316 317 319 319 322 323 323 324 324 325 331 INTRODUCTION Vasculardevelopmentis a central themein aspects of biology as diverse as evolution and cell biology. Ourcurrent conceptionof the control of developmentof this tissue is extremelyrudimentaryin comparisonto the strides whichhave been madein other aspects of biology in recent years. Perhaps this is not surprising considering the complexity and subtleness of the ~Abbreviations used: AT, adenine thymidine; BAP, benzylamino purine; BUdR,bromuridine deoxyriboside; EM,electron microscope; ER, endoplasmie retieulum; FUdR,fluorouridine deoxyriboside; CA, gibberellin; NAA,naphthalene acetic acid; PAL, phenylalanine ammonia-lyase; QC, quiescent center; TdR, thymidine deoxyriboside. Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. 314 SHININGER problem.Thestudy of the control of vascular development,however,offers an openfield of investigationfor the imaginativeinvestigatorinterested in either basic aspects of eukaryotic developmentalbiology in general or in vascular plant physiologyand development specifically. There havebeena numberof reviewsof the subject froma variety of points of view: primary vascular development(29, 30); shoot apical meristembiology (140); apical meristembiology (131); phloemregene.ration(60); eukaryoticdevelopment(135); and xylemdevelopmentin general (101, 102). Thepresent review is an attempt to integrate morerecent workon the developmental biology of vascularization with the earlier physiological and anatomical literature. Thegoal is to identify what wepresently knowabout vascular developmentand to point out directions future workmighttake in order to expandour conceptualization of vascular development. Vasculardevelopment involves first the formationof cells (procambium) which are committedat somepoint to becomexylem and phloemas opposedto pith, cortex, mesophyll,and epidermis. Secondly,it involves the overt cytodifferentiation of these procambialcells into xylem,phloem,and cambium.Whatis the nature of these pote:atial vascular cells whichare currently called procambiumduring primary development or cambium during secondary development?Havethey, at the time at which they becomecytologicallyidentifiable, actually progressedtowarddifferentiation into xylemor phloem,or are they simply ordinary meristematic cells? Answersto these questions can only be obtained whenthe followingquestions are also answered:(a) Whatare the early biochemicalmarkers vascular development? (b) Whatregulates the spatial distribution of vascular cell formation within the stem, leaf, or root? (c) Whatmechanisms operate to prevent the formationof vascular elementsin maturetissues or to stimulate their formationin maturetissues? PROCAMBIUM: DEVELOPMENT IN THE SHOOT The anatomical approach to vascular developmentin shoot apical meristems has generated somecontroversy concerningwhat should properly be called procambium. Until overt cytodifferentiation occurs, however,it is only possibleto define these cells as beinglarger or smaller, denseror less dense, etc relative to surroundingcells. Onecannotdefine these cells in terms of either their developmentalcommitment or their real developmental state. Thus,it seemspresumptiveat this point to state morethan that onecan observesomeof the cytological changesin the progressionof calls towardmaturationas part of the vascular system.As yet, experimentshave not beendonewhichwouldallow one to define the stage at whichthe cells are committedto xylemor phloemdifferentiation unless they haveovertly differentiated. Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. CONTROLOF VASCULARDEVELOPMENT 315 Esau(30) has taken the simplest view that in angiospermsthe residual meristem,a residiumof the shoot apical meristem,extends fromthe region of the apical initials into the differentiating region of the meristemwhere differential parenchymatization of the pith andcortex reveal its existence. Portionsof the residual meristemare consideredto differentiate into procambium,a meristem for the vascular system. Wardlaw(140) takes different point of view based on his workwith ferns. Heobserveda zone of cells whichextends into the apical domeabove the insertion of the youngestleaf pdmordium and whichhe considers to be procambium. In his view, vascular developmentis initiated prior to the developmentof leaf primordia.Thedevelopment of the leaf primordia,in fact, causesdisruption of this initially continuousprocambium, resulting in the formationof parenchymatous leaf gapsin the stem. Surgical removalof the primordiaresulted in eliminationof gapformation(I 39). ThusWardlaw believes that the cells abovethe leaf primordiarepresent procambialcells committedto vascular developmentunless specifically altered by influences from developing leaves. Theinterpretationof the histologicalpreparationsis clearly a subjective matter since one does not knowfrom these kinds of experimental approacheswhether the cells are committedto vascular development,to another fate, or not committedat all. Wardlaw’s viewcan only be accepted whenrigid criteria of procambium are developedand whenthose criteria are successfullyappliedto the cells he observedin the apical domeor when those cells are successfully isolated andinducedto developinto vascular cells in vitro with relatively simpleinput. Experimentalapproachesto the nature of procambium and the role of leaves in vascular developmentwere taken by Helm(50) and Young(146). Bothobservedthat in angiospermsthe removalof the youngleaf primordia caused the already recognizable procambium to develop into parenchyraa and not to developinto vascular tissue nor even remain identifiable as procambium.Furthermore,Young(146) found that supplying auxin in the place of an excised leaf primordium preventedthe changein the appearance of the procambium but did not stimulate its developmentinto vascular tissue. Morerecently, McArthur &Steeves (75) attemptedto clarify questions about the nature of procambium and the effects of leaves in Geurn chloense. In this angiospermthe removalof the leaf primordia did not eliminate leaf gap parenchyma formation within the presumptivevascular cylinder as observedby Wardlaw (139) in ferns, althoughthe cells did not differentiate into parenchyma as observedby Helm(50) and Young(146). McArthur & Steeves (75) concludedthat the early phaseof vascular developmentis not underspecific control of the leaf since "provascular"tissue increasedin longitudinalextent in the absenceof the leaves. In Geurnthey found that vascular developmentprogressed normally whenauxin plus sucrose was applied in the absence of the leaf pdmordinm but not with Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. 316 SHININGER auxin alone. In the absenceof knowledgeof the effect of the auxin plus sucrose in Young’s(146) workwith Lupinus,it is not possible to makeany generalizations concerningthe nature of the stability of provascular or procambialtissues in the absenceof developingleaves. However,from the fact that presumptivevascular tissue did not undergoparenchymatization in Geumwhile it did in Lupinusin the absenceof leaves, it maybe that there are distinct differencesin the regulation of the formationandin the stability of these cells. Procambium is considered to "differentiate" continuously acropeta[ly (e.g. it appears to developfrom differentiated regions towardundifferentiated regionsin the meristem)in the shoot(29, 76, 77, 136). Ball (3) procambium formationto be initiated near a leaf base andto then differentiate both basipetally andacropetally. Esan(30) questionedthis interpretation on the groundsthat it is extremelydi$cult to discriminateprocambium without intimate knowledgeof the pattern of vascular developmentin the given shoot. Again,identification of these cells is a relatively subjective matter. In axillary shoots procambium maydevelop acropetally from the mainshoot to the axillary bud (29, 42), or basipetally fromthe bud to the shoot (44). Thedirection is reported to be basipetal in adventitious buds (140). To state that the procambium is propagatedcontinuouslyacropetally within the meristemwouldbe conceptuallyclearer, because"differentiate" has a specific meaningwhichhas not yet been demonstratedfor procambium. If one accepts that procambium is a meristemproducing the vascular tissues, then it is clear that the continuedfunctioningof this meristemin the shootdependsuponleaf formation.Theeffect of the leaf is in somecases replaceableby auxin or auxin plus sucrose. It :remainsquestionablewhether or not early changesin cellular appearancerepresentobligate changesin the course of vascular development.Withoutspecific characteristics attributable to procambium, e.g. a specific enzymeactivity, protein complement, ete, it is virtually impossibleto adequatelystudy the development of procambium per se, but rather, onemustdeal witth the later stages of vascular element maturation. THE CONTROL OF PRIMARY VASCULAR DIFFERENTIATION IN THE SHOOT Primaryvascular tissue developmenthas been pursued both descriptively andexperimentallyin Coleusby Jacobsand collaborators. Theyreport that in Coleusthe differentiation of the phloemprecedesthe differentiation of the xylemwith the first formedphloemelementsappearing at the base of a leaf, andthendifferentiation proceedsacropetallyinto the leaf andbasipe- Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. CONTROLOF VASCULAR DEVELOPMENT 317 tally to connectwith the maturevascular cells (reviewedin 60). Aday so later the xylemelementsappear in the procambium at the samelevel at whichthe first phloemelementshadearlier differentiated andthe xylemtoo subsequentlydifferentiates bidirectionally. Jacobs& Morrow (61) pursued the questionof the role of the leaf in the inductionof vasculardevelopment andfoundthat the relative rates of auxin productionby the leaf correlated well withthe rates of xylemdifferentiationin the leaf trace. Theyconcluded that auxin limits primaryxylemdifferentiation in the leaf trace of Coleus. Wangermann (138) subsequently demonstratedthat exogenousauxin could replacethe leaf effect in the differentiationof the primaryxylemof Coleus. It is of interest that the primaryxylemin Coleusconsists only of vessels, andthus this workagrees well with the results in Phaseolusof Jost (65), whofound that xylem developmentstopped whenleaves were excised. However, Jost (65) foundthat the application of auxin only restored vessel element differentiation. Leaf removalcaused cessation of xylemfiber differentiation from the cambiumin Xanthium,but vessels developedin normalnumbers(106). NAA restored normalvascular developmentin that case (107). Kinetinwasfirst implicatedin vascular development in intact plants as a result of the studies of Sorokin, Mathur& Thimann(122). Sorokin Thimann (123) concludedthat kinetin wasresponsible for the differentiation response,althoughthe effects of the kinetin stimulationof growthwere not experimentallyseparated from the xylogenesisresponse. Whileit is generallybelieved that the leaf plays a dominantregulatory role in the overt cytodifferentiationof the procambium into vasculartissues, the variouscontributionsofa leafvs the rest of the shoothavenot beenfully revealed. Auxinis one of the principal regulatory agents supplied by the leaf. Althoughsucrosehas beenshownto improvethe auxineffect, it is not likely that this is ordinarily derivedfromthe leaf, becausenet exportation of sucrose from developingleaves is unlikely until after leaves are well beyondthe stages under consideration in early vascular development.It seemsthat elucidation of the other regulatory components in primaryvas¯ cular differentiation could be revealedin a rather straightforwardwayvia meristemculture. PROCAMBIUM: DEVELOPMENTIN THE ROOT Vasculartissue development in the root wasreviewedextensivelyby Torrey (131) andby Esau(30). Asthey haveindicated, the root meristemis a complexexperimental tissue because there are no complications due to terminal appendages.Herethe stages of vascular development occurred in a true linear sequence. The major questions concerning developmentin Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. 318 SHININGER roots have revolved around: (a) the nature of the pattern-forminginfluences, e.g. is it fromwithin the meristemor imposedby maturetissues; (b) the nuclear cytology of procambium developmentinto xylem; and (c) the ultrastructural changesin the cytoplasmassociated with phloemdevelopment.This section deals primarily with the pattern-formingirdtuenees. Asin the shoot, procambium is distinguishedearly fromother tissues in the root by enlargementof cells whichwill constitute the epidermisandcortex. This results in a central procambialcylinder rather than a ring (131). This central cylinder, whoseoutermostboundaryis the pedcycle, eventually differentiates with a variety of flexible arrangements(within and between organisms)into vascular and, in somecases, pith tissue. At a youngstage the cells withinthe central cyclinderdo not haveuniquecytologicalfeatures at the electron microscopelevel whichmightsuggest their early commitmentto different fates (89). In Torrey’sview(131)the ability of the central cylinderto differentiate into vasculartissue with a variety of patterns s~uggeststhat the termprocambiumis appropriate. This interpretation of procambium cannot carry any connotation of a commitment to vascular developrnent and suggests that procambium in the root, as in the shoot, oughtto be considereda meristem within which vascular developmentmaybe induced, as was suggested earlier in this review. Theformationof procambium fromthe apical initials follows a continuous acropetal sequence (reviewed in 131). Procambium developmentinto vascular tissue is markedby the radial enlargementof the future metaxylem vessel elementsvery close to the apical initiMs (10, 11). This produces(in cross-sectional view)a linear array of xylem.(or a triareh or hexarch,etc array of xylem)with phloemdevelopinglaterally to the xylemor between the points of the triarch, hexarch,etc arrays of xylem.Development at any point in time is further advancedin the basipetally located cells producing an axial developmental sequence.Details of overt differentiation in root and shoot systemsat the cytological, cytoehemical,and biochemicallevels are treated together in the next section. Thesequenceof vascular development al]iows for the possibility of pattern induction either by the maturetissues or patterning inherent in the design of the apical meristem. Thoday(126) suggested that the medstem could be autonomous in pattern formation. Biirming(11) performedexperimentswhichwereperhaps overinterpreted to support that concept. Clowes (13) took a moredirect experimentalapproachof surgical manipulation either the apexor the maturetissues and foundan effect on the pattern of vasenlar development (triarela/hexareh, etc) only whenthe apexper se was treated but not whenthe mature tissues were manipulated. Torrey (129, 130) and Reinhard(98) unequivocallydernonstrated that the apex deter- Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. CONTROLOF VASCULARDEVELOPMENT 319 minesthe pattern of vascular development.Theycultured 0.5 mmroot tips (129, 130) or 0.7 mmroot tips (98) aseptically on definedmedium andfound that these very smallroot tips (thoughnot necessarilyother smallpieces of the root) producedroots with vascular calls with normalorganization. Torrey(130) observedthat the numberof xylemstrands (tdarch, hexareh, ere) differentiating in the procambium corrdated well with the diameterof the procambialcylinder. Auxintreatment of these cultured root tips both increased the size of the procambialcylinder and converted the normal triarch xylempattern to hexarch(130). Odhnoff(87)found that GAcaused xylemto differentiate closer to the root tip and concludedthat GAhas a direct effect on xylogenesis. Morerecently Feldman& Torrey (37) found that in Zea mays (cv. Kelvedon)the complexityof the vascular pattern and the size of the QC could be manipulated coordinately. Whenthe QCis small the vascular pattern is simple, and whenit is large the pattern is morecomplex.As a result of changingthe size of the QC,there is a shift in the position of the proximalmeristemfrom whichall of the cells of the axis except the root cap are derived. Feldman(35) found that in corn the vascular pattern evident within 63 to 110 btmof the root cap junction. Heproposedthat changesin the size of the QCshift the point of pattern initiation to areas of the procambium whichdiffer in diameter, and thus moreor less area is actually availablefor the patterninginfluenceto operate. CYTOLOGICAL AND BIOCHEMICAL ASPECTS OF VASCULARDIFFERENTIATION CytologicalAspects Basiccytologicalaspects of overt vascular cell differentiation weresummarized by Esau(30). Thestatus of phloemcytologyspecifically at the light and EMlevels was summarizedby Esan (31) and Cronshaw(17). Torrey, Fosket & Hepler (135), O’Bden(86), and Roberts 002) reviewed aspects of xylemcell differentiation. Thefollowingdiscussionbeginswith an accountof the cytologyof overt vascularcell differentiation andthen proceeds to a moredetailed presentationof aspects whichhavereceivedconsiderable attention regardingmechanisms or possible markersof differentiation. Within the phloemthe sieve elements undergoconsiderable elongation duringdifferentiation fromprocambium (33). Theydevelopa thickenedcell wall whichdoesnot lignify. Sieveareas format rather specific positionsin the wall. Neuberger& Evert(84) considerthe initiation of wall thickening to be an early diagnostic feature. Significant changesin the nucleus or nucleolus havenot been reported except that nuclear degenerationmaybe nearly completedwhenwall thickening and modification are completed. Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. 320 SHININGER Thedevelopment of callose (,8-1,3-glucan)is anotherdiagnostic feature sieve cells. Callosedevelopsas a padcoveringthe developingsieve area. At maturitythe poresof the sieve area are lined with callose, but they are not pluggedin ftmetioningcells. Thereare also early structural changesin the plastids of sieve elements,the mainchangebeingthe formationof a protein crystalloid within the plastid (83). In manyspecies P-proteinformation diagnosticof sieve elementdevelopment (28, 31). P-proteincan be observed in extremdyyoungedls and maybe the first indication of the fate of the cell (32). P-proteinhas beenidentified in phloemsince Hartig’s(49) description of phloem"slime," but it is not present in all speciesand mayexist in phloemcells other than the sieve elements(31, 34). Northcote& Wooding (85) observedP-protein to be boundin membrane whichthey interpre/ted as ERin origin, Sieve elementsare characterized by extensive development of the ER. The cell biology and biochemistry of P-protein development couldbe a fertile area of research. Theinterested reader shouldconsultthe following accounts of phloemdevdopment:(a) root protophloem(33); gymnosperm phloem(82-84); (c) the general reviewby Cronshaw (17). basic features are similar in eachplant systemas well as in callus (144)and hormone-inducedphloemdevelopmentin excised tobacco pith (17). protein structure per se has beenexploredalso (88). Cytological aspects of xylemcell differentiation were reviewed by O’Brien(86) and by Torrey, Fosket&Hepler(135). Regardlessof the origin of the cell (procambium or matureparenchyma),there are several overlapping stages of development.Theproblemis to ascertain whichof these are critical and then to determinehowthey are regulated. Cell expansionis normal in devdopmentfrom procambiumor cambiumbut not pronounced (if it occursat all) in development frommatureparenchyma. Thus,nuclear or nueleolar changes relevant to expansion growth in proeambiummay confusestudies of xylogenesis.This problemshould not occur in xylogenesis in cultured parenchyma.This problemthen becomesone of identifying the time and place of xylemdevelopmentin order to study nuclear changes. Secondarywall deposition and lignification are diagnostic features of xylogenesis.Thecontrol of specific patterns of wall depositionhas received considerableattention (reviewedin 102, 135). Pickett-Heaps(94) observed changesin the distribution of mierotubulesduring xylemdevelopment.He foundthat initially they wereevenlyspacedalong the cell wall, but later they groupedinto bandsand the cell wall thickeningsweredepositedadjacent to these bundles of mierotubules.Pickett-Heaps(94) showedthat the antimicrotubular drug colchicine disrupted the microtubulesand the wall pattern. Cellulosemicrofibril orientation wasalso altered by colchicinein Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. CONTROLOF VASCULARDEVELOPMENT 321 developingxylemcells (51). Howmicrotubulesaffect call wall deposition remains an unsolved problem. Lignindepositionis consideredto be an early aspect of xylogenesisand it accompaniessecondarywall deposition (145). However,as O’Brien(86) pointedout, wereally do not havea stain for lignin at the EMlevel. Thus it is not possible to study very reliably with EMtechniqueshowlignin deposition and wall formation are coordinated. ¯ Whensecondarywall deposition and lignification near completion,portions of the primary wall maybecomehydrolyzed. This is followed or accompanied by protoplast autolysis. Little if anythingis knownabout the control of these processes.At maturitythe water-conducting cells (vessels, traeheids) consist of daboratelyconstructedcells without protoplasts but with variously modifiedwall regions whichallow rather free liquid movement. Asnoted previously, in the root the metaxylem is first detected by the radial expansionof these cells very near the apical initials (131), Large increases in nuclear DNA in these cells werereported (125). Theseobservations generatedthe conceptthat increasedploidy wasbasic to xylemdevelopment (73). Aswill be seen, this correlationis not universalwithina tissue or betweenorganisms. Corsi & Avanzi(15) reported an eightfold increase in the DNA of metaxylemcells of.4lliumceparelative to pericyclecells whichremainedat the 2Clevel. Theyalso reported changesin the DNA/histone ratios, but it is not clear that these wereconsistent or significant. Innocenti&Avanzi(56) interpret their observationsof this systemas follows:Thedevelopingmetaxylemcells first undergochromosome endo-reduplicationvery close to the apical initials. This is followedby amplificationof the nueleolarorganizer region of the DNA (rDNAcoding for rRNA)whichin turn is followed extrusion of the nucleoli. Metaxylem ceils showeda sixfold increase in rDNA (2). This cycle is repeatedin the 2 to 5ramregion fromthe tip (cap included in measurements)and the cells then stop DNA synthesis. During this time the metaxylem cells undergoexpansionfrom 20 ktmin length to 750/zm.Innoeenti (55) reported a decrease in the histone/DNA ratio developingmetaxylem.The workcannot be evaluated adequately since the data werenot presentedexceptas ratios. Similar results werereportedfrom workin trachearyelementdifferentiation in the corn leaf (70, 124). Asthese investigators indicate, these observationsneedcloser examinationin systems moresuitable for biochemicalwork. However,these studies suggest someexperimentalapproachesthat are not readily apparentfrom biochemical methods.Theprimaryconfusingaspect of these studies is the inability to separate events relevant to expansionfromthese relevant to overt xylo- Annual Reviews www.annualreviews.org/aronline 322 SHININGER Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. genesis. Aswill be seen, biochemicalapproacheshavefailed (unnecessarily) to separate replication-relevanteventsfromt~rachearyelementdifferentiation. Biochemical Aspects Thereare a fewstudies of vascular developn~tentat the biochemicallevel. Thornber& Northcote (128) studied changes in carbohydrate and lignin contents of cells developingfromthe cambium.Theresults wereconfusing becauseof the inadequatemethodsfor such studies at that time. Jeffs & Northcote(63) found an increase in the xylose/arabinose ratio in bean callus after 1 to 2 monthsof hormone-stimulated xylogenesis. Newmethods are nowavailable, and such analyses have been elegantly performedon primarycell walls (67) but not related to secondarywall development vascularization. Lignification of secondarycell walls begins very soon after secondary thickening is initiated (52, 145). TheenzymePALcatalyzes the conversion of phenylalanineto cinnamicacid and is a key reaction in the diversion of carbonfromprotein synthesis to lignin synthesis. PALactivity in various tissues is correlatedpositively with vascular development in the plant (105) and in hormone-treatedcallus (104). Most recently Haddon& Northcote (46) followed the time course of PALactivity during vascular, nodule formation in bean callus. In this case the increased PALactivity correlated fairly well with the initiation of xyloglmesis in the early stages. PAL activity declined later whenit wouldseemthat it oughtto remainat least constant. Haddon& Northcote (46, 47) also examinedB-1,3-glucan synthetase activity as a marker of callose formation during phloemdevelopment. B-1,3-glucansynthetase activity followed a time course similar to PAL activity andto sieve cell differentiation.It is ditficult to analyzethis work since hormone-treated replicating (but nondifferentiating)controls werenot examined. Dalessandro& Northcote(21-23) initiated studies of the activities various enzymesinvolved in nucleosidediphosphatesugar reactions which are expected to be involved in carbohydrate metabolismassociated with vascular development.Enzymeactivities were measured:(a) in the cambium,differentiating xylemor differentiated xylemof twoangiospermsand two gymnosperms, or (b) in cultured Jerusalemartichoke tuber tissue whichhormone-inducedvascular developmentoccurred. Enzymeactivity (cpmin product/mgprotein) did not reveal consistent or large differences in the various tissues examinedin ~ TheJerusalemartichoke experiments suffered fromthe lack of adequatecontrols. Annual Reviews www.annualreviews.org/aronline CONTROLOF VASCULARDEVELOPMENT 323 Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. THE CONTROL OF NONPRIMARY DIFFERENTIATION VASCULAR Differentiation from the Cambium As the primaryvascular tissues differentiate from procambium and the tissue ceaseselongation,someof the procambialcells initiate replication in whichthe cell plate is formedin the longitudinalplane. Thesecells are now consideredto be cambium and they will formall of the secondaryvascular cells, e.g. the wood(xylem,cells differentiated interior to the cambium) and phloem(cells differentiated exterior to the cambium). In the stemthe cells betweenthe vascular (fascicular) bundlesmayinitiate replication to form the interfascicular cambium.Whenthis happensthe cambiumbecornes a sheath of meristematiccells. Theinitiation of replication in the fascicular and interfascicular cambium and seasonal reactivation of the cambiaare attributed to factors derivedfromthe shoot (48, 118). Coster(16) suggested that a hormonewas responsible and Snow(118) demonstrated the hormonalnature of the cambialstimulus. Snow(119)effectively replacedshoot stimulation with exogenousauxin. However,auxin is not alwayssufficient (96). Siebers (112-114)studied the activation of the interfascicular cambium ofRicinus communis.Hefound that the activation of this cambium and the differentiation of xylemoccurredin inteffascicular tissue isolated andcultured on simple mediawithout exogenousauxin. However,youngertissue required kinetin (I 13). Heconcludedthat the development of this meristem was determinedat a very youngstage during proeambium developmentand was not dependentupon homogenetieinduction from the fascicular cambium. Generally, studies of vascular developmentfrom the cambium have centered on explorations of the effects of combinations of hormonesor photoperiodon both cambialdivision and differentiation in qualitative terms. Auxinstimulation of cambial division in decapitated plants may result in division and vascular differentiation (119) or cambialdivision without normaldifferentiation (65, 95, 118-121). In somecases normal vascular development maybe elicited by GA(68) or mayrequire auxin plus GA(25, 141). GAtends to be sufficient in rootedtissue but not in isolated stems. Removalof youngleaves and buds from Xanthiumallows cambialactivity to ~ontinue,onceinitiated, but the majorityof the cells (fibers) do not differentiate. However,vessel elements, though smaller than normalin diameter,developin normalnumbers (106). In this case fiber differentiation is restored briefly by allowingone leaf to developacropetal to this region Annual Reviews www.annualreviews.org/aronline 324 SHININGER Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. of the stemor by addingan auxin (NAA) instead (107). Thederivatives the cambium in Xanthiumare not programmed as a result of their cambial origin; rather, there mustbe hormonalinput to the newcells either during cell formationor subsequentto cell formationor both. If the auxin application is delayed,the cells derivedin the meantime fail to respondin terms of fiber differentiation and remainas thin-walled unlignified parenchyma (107). Similar results wereobservedin other systems(6, 53). Experimentally lnduced Vascular Development tissue development mayoccur in portions of the plant other than fromprocambium in root and shoot meristems.Thebulk of the vascular tis~mesare secondaryand produeed by the vascular cambium.However,the literature on secondary vasculartissue formation,althoughextensive,,.reflects a great interest in the alteration of subtle characteristicsof the ditferentiatedcells, e.g. wallthickness, cell length,etc. Verylittle of this literature deals specificallywith the problemof the control of vascularcell differentiation. Theliterature on the carfibiumwas reviewedby Brown(8) and by Phillipson, Ward& Buttertield (93)! While someexperimental work on vascular developmentfrom the cambium will be consideredin this section, the emphasisof this section will be on experimentally induced vascular developmentin explanted parenchymatoustissue, callus, or woundedstems. WOUND-INDUCEDVASCULARIZATION Vascular Vascular regeneration Vascular regeneration in parenehymaof wounded stemswasfirst reportedby Criiger (18). It wasobservedto progressbasipetally around a wound(115), and Janse (62) suggested that a hormonal stimulus wasresponsible, yon KaanAlbest (66) demonstratedthat leaves acropetal to the woundproducedfactors necessaryfor vascular regeneration. She also showedthat the vascular systemper se must be woundedto obtain regeneration. Xylemregeneration mayinvolve the transformationof somecells directly into xylemwithoutcell division (I 17), althoughonedoes not knowwhat unobservedbiosynthetic evenlls maybe prerequisite. Parenehymais not converteddirectly to sieve elementswithoutcell division (66). The major break in understanding vascular regeneration came from Jaeobs’ work(57-59), whichshowedthat the role of the leaf in xylem regeneration wasreplaceable by auxin. Subsequently, La Motte & Jacobs (71, 72) showedthat phloemregenerationwassimilarly controlled. Surprisingly, in viewof studies in other systems,sucrosedid not limit in the Coleus system(59, 72). In Coleusthe leaves needto be present for 48 hours after woundingto obtain a xylogenic response (100). Aninductive effect was evident since the vascular strands did not completedifferentiation until 72 Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. CONTROLOF VASCULARDEVELOPMENT 325 hours after wounding(I). The dependenceof phloemregeneration on roots is variable. Houck&La Motte(54) report that roots are essential but can be replaced by a cytokinin (zeatin). However,previous workhad shown role for the roots in the sameclone of Coleus(59). Thestudies on hormoneinvolvementare, with the exceptionof the auxin studies, in conflict. In additionto the differencesreported concerningthe role of roots and cytokinin noted above, there is a clear indication that kinetin inhibits woundvessel regenerationin a different clone of Coleus (40). In that cloneGAenhancedthe auxin effect on regeneration(103). Thompson (127) found GAto haveno effect on regeneration in the Princeton clone of Coleus. The mannerof hormonepresentation maybe critical because Thompson (127) found that concentrations of auxin which are effective whenapplied apically are not effective in regenerationwhenapplied basally. Ultimately, regeneration is a wound-induced redevelopmentof somemature parenchyma,but it is importantto establish howthese newlyformed cells are "plumbed" into the vascular system.Is the newvascular tissue in the woundregion "plumbed"directly into the old vascular system, e.g. old cells -~ newcells -~ old cells, or is there a moreextensive replacement resulting in the insertion of the regeneratedcells into a newvascularstrand, e.g. newcells -* new(regenerated)cells -~ newcells? Thesequestionswere posed by Benayounet al (5). Theyinterpret their workto support the replacementview, e.g. newcells ~ new(regenerated)cells ~ newcells. their workthe act of woundingactually causes a reduction in the rate of formationof vascular cells. It is probablethat a variety of patterns of regeneration maybe produced(D. E. Fosket, personal communication). Jacobs(60) has reviewedthe earlier workon regeneration. VASCULARIZATIONIN CULTUREDTISSUE Significant progress toward understandingthe control of vascular developmentcamewhenCamus (12) showedthat strands of xylemelementscouldbe inducedto differentiate in the parenchymatous ceils of endivecallus as the result of the insertion of a growingbud. Ball (4) observeda similar effect whenbudswereregenerated in Sequoiasernpervironscallus. Oneof the roles of the inserted bud is to supplyauxin (12). Wetmore &Sorokin(143) performedsimilar experimentswith lilac callus andbudsandfoundthat auxinimitatedthe budeffect in formingxylemstrands only whensupplied froma point source. Theneed for sucrose was demonstratedby Wetmore & Rier (142), and it wasshown in Parthenocissustricuspidata callus that tracheary elementproduction varied quantitatively with changesin sucroseconcentrationwhenauxin was hdd constant (99). Furthermore,the ratio of xylemto phloemvaried with sucrose concentrationif auxin levels were constant: low sucrose (ca 1%) Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. 326 SHININGER elicited relatively morexylemwhilehigh sucrose(ca 4%)elicited relatively more phloem. Thosereports of carbohydrateeffects led to the investigationof effects of alternative carbohydratesupplieswhichare still not satisfactorily resolved. In Phaseolusneither glucose plus fructose nor glucose alone replaced sucrose in producingorganized nodules of xylemand phloem(64). However, glucosealone causedthe productionof scattered xylemelements.Fructose, mannose,xylose, rhamnose,arabinose, galactose, mannitol, or ~t-methyl glucosideat 2%did not elicit anydifferentiatiton, whilecellobiose,lactose, and raflinose (2%)all elicited somexylemdit~:rentiation but not organized nodules of xylemand phloem.Thesucrose elect on noduledifferentiation waspartially replaced quantitatively by 2%maltoseor ,t~-trehalose (64). Sequentialtreatments of sucrose then IAA,or’ IAAthen sucrose, indicated that nodule formation occurred only whenIAApreceded or was accompanied by sucrose (64). Helianthustuberosa tissue forms traeheids i.n responseto NAA and BAP in the presenceof sucrose or glucose or trehalose, or to a lesser extent maltose,whileother carbohydrates are very muchless effective (78). Soluble starch (4%)stimulatedxylogenesisin H. tuberosaquantitatively abovethat produced by optimal (4-8%) sucrose (78). Phillips & Dodds(90) unable to confirmeither that observation or the trehalose stimulation. Minocha& Halpedn(78) observedan inhibition of differentiation without concomitanteffects on cell division whenincreasing amountsof glucose werepresentedwith optimal levels of sucrose. Increasedamountsof glucose or sucrose alone did not have the same effect, but data on the glucose response was not included, Theypostulate: a competitive uptake effect whichcould be tested. Replottingtheir data (percentageof differentiation vs total cell number)indicates that the glucoseeffect maybe a simpleshift of the percentageof differentiated cells eurw~up on the cell numberscale. Whetherthis meansa simpledelayin the initiation of xylemdifferentiation or a decreasein rate of formationof xylemcannotbe deduced.Weare not at a point wherethe specific effects of carbohydratesourceson differentiation can be explainedsatisfactorily. Thesituation is madeevenworseby the inability of investigators to see the sameqmditafiveresponsesin the same tissue. However,it appears that only those carbohydrateswhichsupport significant cell division also supporttraehem~elementformation.Thedisproportionateeffect of carbohydrates on ditSerentiationrelative to cell division should be investigated in a time course experimentto determinehow carbohydratesaffect the initiation andrate of xylogenesis. Therequirementfor auxinobservedin va~;culardifferentiation relative to primary, secondary,or regeneratedvascular cells is also observedconsistently in all formsof tissue culture, e.g. callus (12), pith (14), pea parenchyma (134), Jerusalemartichoke tuber parenchyma (20, 78), or Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. CONTROLOF VASCULARDEVELOPMENT 327 tuce pith (19, 24). Thespecific roles of auxin havenot beendecipheredbut maybe related to aspectsof cell division or geneexpressionor carbohydrate metabolismor all these. Foskct(39) foundthat IAAdid not alter the time lag preceding woundvessel memberformation in Coleus whereexogenous auxinincreases the numbers of vascularcalls formedbut is not essential for their formation. Auxinhas only a small affect on DNA synthesis in Coleus (39) and no affect by itself in pea (116), and so it cannot be assumed increase the numbersof differentiated cells via increasing the numbers availablefor differentiation. It is not clear howauxinaffects RNA or protein synthesis in those systemswhereauxinsuffices to inducevascular differentiation since "state-of-the-art" biochemicalapproacheshavenot beenutilized. Cytokininswerefirst shownto stimulatexylogenesisin culturedtissue in conjunction with exogenousauxin by Bergmann (7). That observation was confirmedin a qualitative mannerin several systemsby Torrey(132). The first quantitative analysis wasachievedby Fosket &Torrey(41), whoused a soybeancallus whichrequired these hormonesfor replication as well. Increasingconcentrationsof kinetin stimulatedxylogenesisrelatively more than replication. Thesoybeansystemusedno longerexists. It is an unfortunate fact that callus cultures are notoriouslycapriciousanddevelopaltered hormoneresponsesas they age. It is essential to developand employadequatemeansof tissue preservationif these studies are to achievetheir true potential in developmental studies. Torrey& Fosket(134) observedsimilar responsesto auxin and cytokinin in pea root parenchyma.This system is knownto contain a heterogeneous cell population. However,xylogenesis occurs in the hormone-stimulated replicating cortical parenchyma (134). Phillips & Torrey(91) refined analysis by punchingout the central vascular cylinder. Theyestablished that xylogenesisandreplication of these cells absolutdyrequiredauxinplus cytokinin andthat replication precededxylogenesisby several days. Shininger &Torrey(111)then establishedthat, like the soybeansystem,increasing concentrationsof eytokininstimulatedthe rate of xylemcell formation relatively morethan cell replication. Furthermore,these cells showeda continuousrequirementfor the hormonesfor xylogenesiswhile cell divisions wereinducedby shorter periods of cytokinin treatment and continued after cytokinin "removal."This maynot be true in the Jerusalemartichoke tissue since preliminary worksuggestedthat xylogenesiscould continue after cytokininwithdrawal(78). Cytokininis required in lettuce pith (19), carrot tissue (80), and Zinniamesophyll(69) as well as pea (91, 111, 134) andartichoke(78). Thespecific role of cytokininremainsto be determined in all systems. Cell replication is observedto precede and accompany xylogenesis in nearly all culture systems.In those cases wherereplication is not observed Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. 328 SHININGER it has not beenshownthat DNA synthesis did not occur. Withinthe plant, cell division normallyaccompaniesprimarymadsecondaryvascular developmentas well as wound-inducedor hormone-inducedvascular devdopment. Onrare occasionstracheary elementformationcan also be observed in single calls in suspension cultures, but in general,replicationis coincident (133). Dothese observedcell divisions haveany bearing on the problem the regulation of vascular differentiation? I think they do. In animal systems a considerable body of evidence has developed to supportthe conceptthat someformsof differentiation require cell replication (reviewedin 97). Rigorousevidencein plant systemsis minimaland, like animalsystems,dependsuponthe use ofitthibitors (reviewedin 102and 109). In plants the first direct approachto the questionwasfocusedon the need for cell division in toto. Fosket (38) found that both FUdR and mitomycin C blockedreplication and xylogenesisin Coleusexplants. TheFUdReffect was prevented by simultaneousTdRtreatment. This indicates a need for someaspect of the division process. Althoughcolchicinehad similar effects in this system,it is less clear wherethe effect wasmanifest(D. E. Fosket, personal communication).Turgeon(137) concludedthat DNA synthesis not necessary for vascular differentiation, :~inee FUdRdid not prevent xylogenesisin lettuce pith. This interpretation maybe prematurebecause it was not shownrigorously that DNA synthesis wasprevented. It is not adequateto demonstratea lack of increase i~n cell numbersor to measure DNAmicrospectrophotometrieally in 0.5%of the cells (when 6%will differentiate) andconcludethat DNA synthesis wastotally blocked.In fact, FUdR had a pronounced effect on vascular ,differentiation since it reduced vascular differentiation to 3%of the control level. Theevidencefromgamma plantlets (45) is frequently used to support the viewthat vascular development does not require recent cell divisions. However, recent cell divisions haveonly beenconsideredessential as a result of studies whereit is knownthat they are occurringcoincidentlyin time with the agents whichactually induce differentiation. It is not knownwhen induction takes place relative to overt cytodifferentiation in the gamma plantlets. Wedo not yet knowwhyreplication is essential althoughthe pea systemis yielding somenewinsight. Endopolyploidyis frequently observedto be correlated with in vivo xylogenesis(73). Phillips & Torrey(92) andDodds&Phillips (26) carefully eliminatedthis possibility as beinga causal relationshipin pea andJerusalemartichoke respectivdy. However,the needfor normalreplication in the pea system remains. Shininger(108) demonstrateda reversible FUdRinhibition of xylogenesis in the pea system, and with the use of BUdR(a thymidine analog) Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. CONTROL OF VASCULAR DEVELOPMENT 329 demonstrateda specific role of DNAsynthesis per se in the differentiation process. BUdRdid not block cell replication. Both FUdRand BUdReffects are prevented and reversed by simultaneous or subsequent TdRtreatment. Both replication and xylogenesis were blocked within 48 hours of FUdR treatment and both recovered within 72 hours of TdRaddition (108). How BUdRelicits its effect is unknown. BUdRincorporation into DNAis considered essential (74). BUdRis incorporated into the DNAof pea cells (108). In Tetrahymena BUdRblocks RNAsynthesis in synchronized ceils but only if it is available to cells during DNA synthesis and most specifically during replication of the ribosomal genes (74). Lykkesfeldt &Andersen(74) postulated that this BUdR effect results from its incorporation into AT-rich regions. Thus, the transcription of all AT-rich regions maybe selectively inhibited, and this could conceivably block RNAsynthesis related to xylogenesis but not that whichis necessary for cell replication. Factors other than hormoneshave not been extensively investigated relative to their use in studies of vascular development. Increased pressure stimulates xylemdifferentiation in stems (9). The mechanismhas not been investigated, but Roberts (102) suggested it occurred through induced ethylene production. Doley & Leyton (27) found lowered water potential stimulate xylogenesis and suggested that this might be the basis of the observed sucrose stimulation. Other investigators have not found osmotic agents in general to stimulate vascular developmentas efficiently as sucrose. In Jerusalem artichoke tuber tissue it was observed that (a) reducing the nitrogen concentration of the medium, and (b) reducing the volume mediumare both stimulatory to xylogenesis (90). The volumeeffect does not seem due to a "conditioning" of the medium(90). Light generally inhibits xylogenesis. In the Jerusalem artichoke, a brief exposureto dim white light inhibited cell replication and xylogenesis relative to a dim green light control (90). Continuouswhite light (relative a brief exposureto white ligh0 had little effect on replication but inhibited xylogenesis (90). Similar results have been observed in the pea system (T. L. Shininger, unpublished observations). In carrot culture, light can be requirementfor xylogenesis (79-81), but if so, it is replaceable by cytokinin (79). Studies of the light effect in other systems need to be conductedmore conscientiously. Temperature as a probe of vascular development has not been utilized generally. Gautheret (43) reported that in the Jerusalem artichoke callus temperatures less than 17°C were inhibitory. This was confirmed in freshly excised tubers as well (90). Naik (82) reported that a high temperature (35°C) stimulates xylogenesis in this system, but this was not confirmed subsequent work (90). In any case, these effects have not been distinguished from effects on replication. Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. 330 SHININGER In a recent series of experimentswith the pea system, I havefoundsome specific effects of temperatureon xylogenesis(110). Briefly, low (10°C) high (30°C)temperaturesinhibit xylogenesisrelatively morethan replication. Lowtemperature wasstudied extensivdy, and it wasfound to delay the initiation of xylemcell appearanceby several days. However,once initiated, these cells appearedat essentially normalrates. Cell replication wasnot affected in this ease. This is not causedby an affect on overt cytodifferentiation becausetransfer of cells to low temperatureafter the initiation of xylogenesisdid not cause a drop in the rate of xylemcell appearancefor at least 7 days. When cultures wereinitiated at lowtempera. ture and then shifted up, there was no transient increase in the rate of vascular cell appearanceto suggest they had accumulatedat a specific temperature-sensitivepoint in development.]Brief low temperaturetreatments(24, 48 hours, etc) indicated that xylogenesiswastemperaturesensitive before replication of the cortical parenchyma wasinitiated. Theresults suggest that since xylogenesis can be uncoupledfrom the early roundsof replication, these early replications haveno uniquerole in development. Sinceit is unlikelythat cells committed to xylogenesisexist in the cortical parenchyma,it follows that xylogenesisin this system can be inducedby definedexogenous stimuli whenever the cells ~Lrereplicating at an appropriate temperature. °Thepea systemoffers someinteresting points for further analysis. At 25 both xylogenesis and nonxylemcell formatiion shownearly exponential kinetics. Howthis relates to the fate of individualreplicatingcells is not yet known.The possibilities are that one cell provides two daughters which differentiate or that onecell providesonecell for continuedreplication and one for vascular developmentor somecomplication of this scheme.Observations of sections havenot yet revealed isolated single tracheary elements, but it is prematureto state that they are not to be found in this system. It is also interesting to note that in this systemonecan observelinearity betweenthe log of the vascular cell numberand log of the nonvascular cell number.This relationship is not disturbed by temperaturechanges.Nor is it altered by changesin cytokininconcentrationwhichare known to affect the rates of xylemand nonxylemcell formation differentially. This may meanthat the rates of these processesare coupled.If so the result of this is that chan~esin replication rates generatetissues with different percentages of vascular elementsrather than a proportionateincrease in the number of xylemand nonxylemcells. The log/log relationship is disturbed by BUdR.Myreanalysis of other systemsindicates that whereadequatedata exist the samerelationship occurs(41, 90). Exactlyhowthese responsesare coupledremainsto be investigated. It seemsclear that this relationship indicates a basic developmentalcontrol mechanism. Annual Reviews www.annualreviews.org/aronline CONTROLOF VASCULARDEVELOPMENT 331 Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. CONCLUSIONS: Control of Vascularization in the Shoot and Root Theprocessof vascularizationinvolvesfirst the productionof a population of cells in whichvasculardifferentiation can be induced.Theprocessought to be viewedas a continuumof developmentalevents involving as a first identifiable step the distinguishingof the potential vascularcells fromthe nonvascularby differential parenchymatization.Thenonparenchyma cells maynowbe considered to be in the pathwayto vascular developmentbut are not obligatedto that fate becauseremovalof exogenousstimuli (auxin, sucrose) allows themto divert to the parenchyma state. Procambium is term that shouldbe employed to describe potential vascular cells whichare at this state of development. It shouldbe possible to further breakdownthe stages of differentiation within this concept of procambium.Whenthese cells irreversibly changecytologically toward either the xylemor the phloem,they can be consideredovertly differentiated and no longer procambial. In shoots, stimuli are derived in part fromthe developingleaves and in part fromthe matureshoot. In the root these factors maybe derived in part from the terminal portions of the meristem(root cap, QC,and proximalmeristem)and in part from moremature sections of the root. In the shootit is clear that the leaves play an impo~antregulatory role in the formationand development of procambium as it is currently identified. However,we do not have a meaningfulconcept of procambium at a biochemicalor ultrastruetural level, and the development of concepts at these levels is important.Bothauxin andsucroseare critical to procambium formationandto the formationof vascular cells in cultured tissue. Wedo not knowthe roles of either of these agents in the differentiation process. Overtdifferentiation probablycan be monitoredat the biochemicallevel andcertainly can be monitoredat the ultrastructural level. It is conceivable that phloemdifferentiation could be followed immunologieallyin those organismswhichform P-protein or biochemieallywith careful analyses of fl-1,3-glucansynthetaseactivity in conjunctionwith cytologicalstudies. It is not clear if significant nuclearor nucleolarchangesoccurin sieve element differentiation. Thechangesin the nuclei of differentiating trachearyelements mayhave been misleading, however. Biochemicalanalyses of tracheary elementdifferentiation maybe more ditiieult until secondarycell wall compositionis characterizedin greater detail. Lignifieation probably can be used as a valid marker of xylem differentiation, especially since onereally wantsa markercharacteristic of terminalaspectsofcytodifferentiation.Thereexists the possibility that there is considerablymoremicrotubularprotein in differentiating xylemcells if microtubulesare really associated with secondarywall deposition. Sucha quantitative changemightbe detectable. Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. 332 SHININGER DNA synthesis seemscritical to xylemdifferentiation. Withbiochemical markersof xylogenesis,it maybe possible to determinethe reason for the need for DNA synthesis. Thevarious roles of auxins, cytokinins, and occasionally gibberellins need meaningfulexploration. Wedo not have an adequate understanding of these hormoneseven in simpler response systems. These hormonescan only be understoodwhenplant scientists generally becomewilling to explore hormoneresponses with truly rigorous and contemporarybiochemical or genetic approaches.The exploitation of temperatureand light may be useful and, becauseof the "neatness" of such experiments,the results might be extremely important. Thedevelopment of suspensioncultures that will differentiate reasonably synchronouslywouldbe extremelyuseful. Therecent workof Torrey (132) makesone optimistic commentabout that approach. The recent manipulationsof the QCeffect on developmentand attempts at physiological replacement of the QCwidth hormonesboundto resin coated beads (36) provide impetusto explore these systemsmoreimaginatively. Finally, the autonomous nature of t!he root tip makes it ideal for exploration of manyof the sorts of questions whichpreviously have been consideredonly in tissue culture. Thechief advantageof the root tip culture is the productionof vascular cells in predictable time and space. Thusit ought to be possible to define procambium in the root in terms of its responses to specific metabolic analogs or physical treatments in ways analogousto the early workof Torrey(132,). Literature Cited 1. Aloni,R., Jacobs, W.P. 1977.Thetime courseof sieve tube andvessel regeneration and their relation to phloemanastomosesin matureinternodesof Coleus. Am. J. Bot. 64:615-21 2. Avanzi,S., Maggini,F., Irmocenti, A. M. 1973. Amplilieation of ribosomal eistrons during the maturation of metaxylem in the root of Alliumcepa. Protoplasma76:197-210 3. Ball, E. 1949. Theshoot apexand norreal plant of Lupinusalbus L. bases for experimentalmorphology.Am. J. Bot. 36:4~)-54 4. Ball, E. 1950.Differentiationin a callus culture of Sequoiasempervirens.Growth 14:295-325 J., Aloni,R., Sachs,T. 1975. 5. Benayoun, Regeneration around woundsand the controlof vasculardifferentiation.Ann. Bot. 39:447-54 6. Benayoun,J., Sachs, T. 1976. Unusual xylemdifferentiation belowa mature leaf of Melia.Isr. J. Bot. 25:184-94 7. Betgmann,L. 1964. Der Einfluss yon Kinetin auf die Ligninbildung und Differenzierung in Gewebekulturen yon Nicotiana tabacu~ Planta 62:221-54 8. Brown,C. L. 1975. Secondarygrowth. In Trees, Structure and Function,ed. M. H. Zimmermann,C. L. Brown, pp. 67-123. NewYork: Springer-Verlag 9. Brown,C. L., Sax, K. 1962. Theirdlueneeof pressureonthe differentiationof secondary tissues. An~J. Bot. 49: 683-91 10. Biirming,E. 1951. 0her die Differen. zierungs-vorg’fingein der Crueifemwnrzel. Planta 39:126-53 11. Bilnning, E. 1952. Weitere Untersuchungenfiber die Differenzierungsvorg’~ingein Wurzeln.Z, Bot. 40:385-406 Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. CONTROL OF VASCULAR DEVELOPMENT 333 12. Camus,G. 1949. Recherehessur le r61e cultures of Acerpseudoplatanu~ J. Exp. des bourgconsdans les ph6nom~nes de Bet. 17:718-25 morph6g~ese(Extrait). Rev. CytoL 26. Dodds,J. H., Phillips, R. 1977. DNA Biol. Veg. 11:1-199 and histone content of immaturetrac13. Clowes,F. A. L. 1953. Thecytogenerahearyelementsfrom cultured artichoke explants. Planta 135:213-16 tire centre in roots withbroadcoinmellas. NewPhytoL52:48-57 27. Doley,D., Lcyton,L. 1970. Effects of 14. Clutter, M. E. 1960. Hormonalinducgrowthregulating substancesand water tion of vasculartissues in tobaccopith potential on the developmentof wound in vitro. Science132:548-49 callus in FraMnu~ NewPhytoL 69:8715. Corsi, G., Avanzi,S. 1970.Cytochemi102 cal analyseson cellular differentiation 28. Esau, K. 1947. A study of somesievein the root tip of Alliumcep~Carylogia tube inclusions. AmJ. Bet. 34:224-33 23:381-94 29. Esau, K. 1954. Primaq~ vascular differentiation in plants. Biol. Rev. 16. Coster, Ch. 1927. Zur Anatomicund Physiologie der Zuwachszonen--und CambridgePhiloz Soc. 29:46-86 Jahresringbildungin den Tropen./lnn30. Esau,K. 1965. VascularDifferentiation Jard. Bet. Buitenzorg37:49-160 in Plants NewYork: Holt, Rinehart & 17. Cronshaw, J. 1974.PhloemditfercntiaWinston.160 pp. tion and development.In Dynamic 31. Esan, K. 1969. The phloem. In Handpects of Plant Ultrastructure,ed. A. W. buchderPflanzenanatomie. Vol. 2. BerRobards, pp. 391-413. London-New lin: Gebr[iderBorntraeger.505 pp. York: McGrawHill 32. Esau, K., Gill, R. H. 1970. Ob~rva18. Crfiger, H. 1855. ZurEntwickelunggestions on spiny vesicles andP-proteinin chichte der Zellenwand.Bet. Ztg. 13: Nicotiana tabacum Protoplasma 69: 601-29 373-88 19. Dalessandro, G. 1973. Hormonalcon33. Esau,K., Gill, R. H. 1972.Nucleusand trol of xylogenesisin pith parenchyma endoplasmicreticulum in differentiatexplants of Lactuca. Ann. Bet. 37: ing root protophloem of Nicotianataba. 375-82 cure Ultrastruct. Res, 41:160-75 20. Dalessandro,G. 1973. Interaction of 34. Evert, R. F., Eschrich,W.,Eichhorn,S. auxin, cytokimn,andgibbcrellinon cell E. 1973.P-protein distribution in madivision and xylemdifferentiation in ture sieve elementsof Cucurbitamaxcultured explants of Jerusalemartiim~ Planta 109:193-210 choke. Plant Cell Physiol. 14:1167-76 35. Feldman,L. J. 1977. The ganeration 21. Dalessandro, G., Northcot¢, D. H. and daborationof primaryvasculartis1977. Changesin enzymicactivities of sue patterns in roots of Zea~Bet. Gaz. nuclensidediphospbatesugar intcrcon138:393--401 versions duringdifferentiation of cam- 36. Feldman,L. J. 1978. Cytokininbiosynbiumto xylemin sycamoreand poplar. thesis in corn roots. Plant Physiol. BiochemJ. 162:267-79 Suppl. 61:11 22. Dalessandro, G., Northcote, D. H. 37. Feldman,L. J., Torrey,J. G. 1975.The 1977. Changes inenzymic activities of quiescent center and primaryvascular nucleoside diphosphate sugar intercon- tissue pattern in cultured roots of Zea versions during differentiation ofcammayz Can. J. BoA53:2796-2803 biumtoxylem inpine andfir. Biochem 38. Fosket, D. E. 1968:. C¢11division and £ 162:281-88 the differentiation of wound-vessel 23.Dalessandro, G.,Northcote, D. H. membersin cultured stem segmentsof 1977. Changes inenzymic activities of Coleus, Prec. Natl. Acad. ScL USA UDP-D-glucuronate decarboxylase and 59:1089-96 UDP-o-xylose 4-epimerase during cell 39. Fosket, D. E. 1970. Thetime course of division andxylem differentiation in xylemdifferentiation andits relation to cultured explants ofJerusalem artiDNAsynthesis in cultured Coleusstem choke. Phytochemistry 16:853-59 segments.Plant Physiol. 46:64-68 24.Dalessandro, G.,Roberts, L.W,1971. 40. Fosket, D. E., Roberts,L. W.1964.InInduction ofxylogenesis inpith parenductionof woundvessel differentiation chyma explants ofLactuca. Am.LBoA in isolated Coleusstemsegmentsin vi58:378-85 tro. Amd. Bet. 51:19-25 25.Digby, J.,Wareing, P.F. 1966. The 41. Fosket, D. E., Torrey,~. G. 1969.Horeffect ofgrowth hormones oncell divimonalcontrol of cell proliferation and sion andexpansion inliquid suspension xylemdifferentiationin culturedtissues Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. 334 SHININGER of Glycine max vat Biloxi. Plant Physiol. 44:871-80 42. Garrison, R. 1949. Origin and devdopmentof axillary buds, Syringavulgaris L Am-J. Bot. 36:205-13 43. Gautheret,R. J. 1961.Actionde la lumitre et de la temperaturesur la n~oformation de racines par des tissues de Topinambour eultives in vitro. C. R. Acad. ScL 250:2791-96 44. Gregory, F. G., Veale, J. A. 1957. A re-assessmentof the problemof apical dominance.Soc. Exp. Biol. Symp.1 h 1-20 45. Haber,A. H. 1968. Ionizing radiations as research tools. Ann. Rev. Plant Physiol. 19:463-89 46. Haddon,L. E., Northcote, D. H. 1975. Quantitative measurement of the course of beancallus differentiation.J. CellSci. 17:11-26 47. Haddon,L., Northcote, D. H. 1976. Theinfluence of gibberellic acid and abseisieacid oncell andtissue differentiation of beancallus. J. Cell Sci. 20: 47-55 48. Hartig, T. 1853. Uberdie Entwieklung des Jahringes der Holzpflanzen.Bot. Ztg. 11:553--60 49. Hartig, T. 1854. 0ber die Querscheidewiindezwisehenden einzeinen Gleidern der SiebrShren in Cucurbita pepo. BoAZtg. 12:51-54 50. Hdm,J. 1932. 0ber die Ikeinflussungder Sprossgewebe-Differenzierung durch Enffernender jungen Blattanlagen. Planta 16:607-21 51. Hepler, P. K., Fosket, D. E. 1971. The role of mierotubulesin vessel member differentiation in ColeuzProtoplasma 72:213-36 52. Hepler,P. K., Fosket, D. E., Neweomb, E. H. 1970.Ligniticationduringsecondary wall formationin Coleus:An dectron microscopic study. Am.J. Bot. 57:85-96 53. Hess,T., Sachs, T. 1972.Theinfluence of a matureleaf on xylemdifferentiation. NewPhytol. 71:903-14 54. Houek, D. F., La Motte, C. E. 1977. Primary phloemregeneration without concomitant xylem regeneration: Its hormonecontrol in Coleus~Am-J. Bot. 64:799-809 55. Innoeenti, A. M. 1975. Cyclic changes of histones/DNA ratio in differentiating nuclei of metaxylem cell line in AIlium cepa root tip. Caryologia 28: 225-28 56. Innocenti, A. M., Avanzi, S. 1971. Some cytologicalaspectsof the differen- tiation of metaxylem in the root of AIlium cepa. Caryologia24:283-92 57. Jacobs, W.P. 1952.Therole of auxinin the differentiation of xylemarounda wotmd.Am. J. Bot. 39:301-9 58. Jacobs, W. P. 1954. Acropetal auxin transport and xylem regeneration--a quantitative study. Am-Nat. 88:327-37 59. Jacobs, W. P. 1959. Whatsubstance normally controls a given biological prooess?1. Formulationof somerules. Dev.Biol. 1:527-33 60. Jacobs, W.P. 1970. Regenerationand differentiation of sieve tube elements. lnt. Rev. Cytol. 28:239-73 61. Jaeobs, W. P., Morrow,I. 1957. A quantitative study of xylemdevelopmentin the vegetative shoot apex of Coleus. Am.J. BoA44:823--42 62. Janse, J. M.1921.La polarit~ des cellules cambiennes.Ann.Jard. Bot. Buitenzorg 31:167-80 63. Je~;, R. A., Northcote,D. H. 1966. Experimentalinductionof vascular tissue in an undifferentiatedplant callus. Biochem. J. 101:146-52 64. Jeff.,;, R. A., Northcote,D.H. 1967.The influence of indol-3yl acetic acid and sugaron the patternof induceddifferentiation in plant tissue culture. J. Cell Sc£ 2:77-88 65. Jost, L. 1939. Zur Physiologic der Geff~sbildung. Z. Bo~35:114-50 66. Ka~nAlbest, A. yon. 1934. Anatomisthe und physiologische Untersuchungen fiber die Entstehungyon SiebriShrenverbindungen.Z Bot. 27:1-94 67. Keegstra, K., Talmadge,K. W., Bauer, W.D., Albersheim,P. 1973. Thestructure of plant cell walls. Plant Physiol. 31:188-96 68. Kie:rmayer, O. 1959. Gesteigerte Xylem,mtwieklungbei Solanura nigrum dun~hEinfluss yon Gibberellin~ure. Bet. Dtsch. Bot. Ges, 72:343-48 69. Koldenbach,H. W., Sehmidt, B. 1975. Cytodifferenzierung in Form einer direkten Umwandhmg isolieiter Mesophyllzellenzu Tracheiden.Z. Pflanzenphysiol. 75:369-74 70. Lal, V., Srivastava,L. M.1976.Nuclear changesduring differentiation of xylem vessel elements.Cytobiologia12:220-43 71. La iMotte, C. E., Jacobs, W.P. 1962. Quantitative estimation of phloemregenerationin Coleusinternodes. Stain Tec~nol. 37:63-73 72. La Motte, C. E., Jacobs, W.P. 1963. Therole of auxin in phloemregeneration in Coleusinternodes. Dev. Biol. 8:80-98 Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. CONTROL OF VASCULAR DEVELOPMENT 73. List, A. Jr. 1963.Someobservationson DNAcontent and cell and nuclear volume growth in the developing xylem cells of certain higher plants. ,4~ J. Bot. 50:320-29 74. Lykkesf¢ldt, A. E., Andersen,H. A. 1975.Inhibition of rRNA synthesisfollowingincorporation of 5-bromodeoxyuridine into DNAof Tetrahymena pyriformig J. Cell Sc~ 17:495-502 I. C. S., Stccvcs,T. A. 1972. 75. McArthur, An experimental study of vascular differentiation in Geum~chloense. Bot. Ga~ 133:276-87 76. McGahan, M. W. 1955. Vascular differentiationin the vegetativeshootof Xanthiumchinense. ,4r~ J. BoA 42: 132-40 77. Millington,W.F., Gunkel,J. E. 1950. Structure and development of the vegetativeshoot of Liriodendron tulipifera. d~ J. Bot. 37:326-35 ~ 78. Minocha,S., Halperin, W.1994. Hormonesand metabolites whichcontrol tracheld differentiation withor without concomitanteffects on growthin cultured tuber tissue of ttelianthus tuberosa L. Planta 116:319-31 79. Mizuno,K., Komamine, A. 1978. Isolation andidentificationof substancesinducingformationof tracheary elements in cultured earrot-root slices. Planta 138:59-62 80. Mizuno, K., Komamine,A., Shimokoriyama,M. 1971.Vesselelementformationin cultured carrot-root phloem. Plant Cell Physiol. 12:823-30 81. Mizuno, K., Komamine,A., Shimokoriyama,M. 1973. Isolation of substances inducingvessel elementformation in cultured carrot root slices. In Plant GrowthSubstancegpp. Ill-18. Proc. 8th Int. Conf. Plant GrowthSubstances. Tokyo:Hirokawa 82. Naik,G. t3. 1965.Studieson the effects of temperatureon the growthof plant tissue cultures. MScthesis. Univ.Edinburgh,Scotland(cited in Ref. 90) 83. Neuberger,D. S., Evert, R. F. 1974. Structureand development of sieve.element protoplast in the hypocotyl of t~’nus resinosa~,4rr~ J. Bot. 61:360-74 84. Neuberger,D. S., Evert, R. F. 1976. Structureanddevelopment of sieve cells in the primaryphloemof I~nusresinosa. 2°?otoplasma87:27-37 85. Northcote, D. H., Wooding,F. B. P. 1966. Developmentof sieve tubes in Acerpseudoplatanus. Proc.R. Soc. Ser. B 163:524-37 86. O’Brien,T. P. 1974. Primaryvascular tissues. SeeRef. 17, pp. 414--40 335 87. Odnoff,C. 1963.Theeffect of gibberellin and phenylboric acid on xylem differentiation andepidermalcell elongation in bean roots. Physiol. Plant. 16:474-83 88. Parthasarathy, M.V., Miiklethaler, K. 1969.Ultrastruetureof protein tubules in differentiatingsieve elements.Cytobb ologie 1:17-36 89. Phillips, H., Torrey,J. t3. 1974.The ultrastructureof the quiescentcenterin the apexofL.cultured of Convolulus arvensis Am.J. roots BoL61:871-78 90. Phillips, R., Dodds,J. H. 1977. Rapid differentiation of trachearyelementsin cultured explants of Jerusalemartichoke. Planta 135:207-12 91. Phillips, R., Torrey, J. ~3. 1973. DNA synthesis, cell division and specific cytodifferentiationin cultured pea root cortical explants. Dev.Biol. 31:336-47 92, Phillips, It., Torrey, J. G. 1974. DNA levels in differentiating trachearyelements. Dev.Biol. 39:322-25 93. Phiilipson, W.R., Ward,J. M., Butterfield, B. ~. 1971. The Vascular Cambium: Its Developmentand Activity, London: Chapman&Hall. 182 pp. 94. Pickett-Heaps, J. D. 1967.Theeffects of colchicineon the ultrastructure of dividingplant cells, xylemwall differentiation anddistribution of cytoplasmic mierotubules.Dev.Biol. 15:206-36 95. Rehm,S. 1936. Zur l~ntwicklungsphysiologie der Geffisse und des traehealen Systems.Planta 26:255-74 96. Reinders-g3ouwentak, C. 1965. Physiology of the cambium and other secondary medstemsof the shoot. Handb. PflanzenphysioL15:1077-1105 97. Reinert,J., Holtzer,H., eds. 1975.Cell Cycle and Cell Differentiation. New York:Springer. 331 pp. 98. Reinhard, E: 1954. Beobaehtungenan in vitro kultivierten bewebenaus dem Vegetationskegelder PisumWurzel.Z. Bot. 42:353-76 99. Rier, J. P., Beslow,D. T. 1967.Sucrose concentrationandthe differentiation of xylemin callus. Bot. Gaz.128:73-77 100. Roberts, L. W. 1960. Experimentson xylemregeneration in stem woundresponses in ColeuxBot. Gaz.121:201-8 101. Roberts, L. W.1969.Theinitiation of xylemdifferentiation. Bot. Rev. 35: 201-50 102. Roberts, L. W.1976. Cytodifferentiation in Plantx Cambridge Univ. Press. 160 pp. 103. Roberts, L. W.,Fosket, D. E. 1966.Interactionof gibberellieacid andindoleaeetic acid in the differentiation of Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. 336 SHININGER woundvessel members. NewPhytol. 117. Sinnott, E. W., Bloch, R. 1945. The 65:5-8 cytoplasmicbasis of intercellular pat104, Rubery, P. H., Fosket, D. E, 1969. terns in vasculardifferentiation. Am.J. Changes in phenylalanine ammoniaBot. 32:151-56 lyase activity duringxylemdifferentia- 118. Snow,R. 1933. Thenature of the camtion in Coleuaand soybean. Planta bial stimulus. NewPhytol. 34:288-96 87:54--62 119. Snow,R. 1935. Activation of cambial 105. Rubery,P. H., Northcote, D. H. 1968. growthby pure hormones,NewPhytol. Site of phenylalanine ammonia-lyas¢ 34:347--60 activity andsynthesis of lignin during 120. S~5ding,H. 1936.Uberden Einfluss yon xylem differentiation. Nature 219: Wuehstoffanf das Dickenwachstum der 1230-34 B~ume.Bet. Dtsch. Bot Gez 54:291106. Shininger, T. L. 1970. Theproduction 304. and differentiation of secondaryxylem 121. S~ling, H. 1940. Weiter¢Untersuchunin Xanthiumpennsylvanicun~Am. J. gen. fiber die Wuchestoffregulation der Bot. 57:769-81 Kambiumt~tigkcit.Z. Bot. 36:8-141 107. Shininger,T. L. 1971.Theregulationof 122. Sorokin, H. P., Mathur, S. N., Thicambial division and secondaryxylem mann,K. V. 1962.Theeffects of anxins differentiation in Xanthiumby auxins and kinetin on xylemdifferentiation in and gibberellin. Plant Physio[ 47: the pea epicotyl. Am.J. Bot 49:444-54 417-22 123. Sorokin, H. P., Thimann,K. V. 1964. synthe108. Shininger, T. L. 1975. Is DNA Thehistological basis for inhibition of sis required for the induction of axillary buds in Pisumsativumand the different/at/onin qu/escentroot cortical effects of auxins and kinetin on xylem parenchyma?Dev. Biol. 45:137-50 development.Protoplasma59:326-50 109. Shininger, T. L. 1978.Hormone regula. 124. Srivastava, L. M., Singh, A. P. 1972. tion of developmentin phnt cells. In Certainaspects of xylemdifferentiation Vitro 14:31-~0 in com. Camd. Bot. 50:1795-1804 110. Shininger,T. L. Quantitativeanalysisof 125. Swift, H. 1950. Theconstancyof deoxtemperatureeffects on xylemand nonyribose nucleic acidSc~ in USA plant nuclei. xylem cell formation in cytokininProc. Natl. dcad. 36:643-54 stimulatedroot tissue. Proc. Nail Aead. 126. Thoday,D. 1939. Theinterpretation of Sc£ USA.In press plant structure. Rep.Br. Assoc.Adv. 111. Shininger, T. L., Torrey, J. G. 1974, 1:84-104.Cited in Ref. 131 Theroles of eytokiniusin the induction Thtompson, N. P. 1965. Theinfluence of of cell division andcytodifferentiation 127.auxin on regeneratfonof xylemand sieve in pea root cortical tissue in vitro. In tubes arounda stem wound.PhDthesis. Mechanismsof Regulation of Plant Princeton Univ., Princeton, N~ Growth,ed. R. U Bideski, A. R. Fergu. son, M.M. Cresswell,pp. 721-28.Wel- 128. Thornber,J. P., Northcote,D. H. 1961. Changesin the ch¢micalcompositionof lington: Bull. 12 R. Soc.N.Z. a cambialcell duringits differentiation 112. $iebers, A,M.1971.Initiation of radial into xylemandphloemtissue in trees. polarity in the inteffascicular cambium Biochem. £ 81:449-55 of Ricinus communis U Acta Bot. 129. Torrey,J. G. 1954.Therole of vitamins Neerl. 20:211-20 and micronutrient elementsin the nu113. Siebers, A. M.1971.Differentiation of trition of the apical meristemof pea isolated inteffasciculartissue of Ricinus communigActa BoA NeerL 20:343-55 roots. Plant PhysioL29:279-87 114. Siebers, A. M. 1972. Vascular bundle 130. Torrey, J. G. 1955. Onthe determination of vascularpatterns duringtissue differentiation and eambial developdifferentiation in excisedpea roots. Am. mentin cultured tissue blocks excised J. Bot. 42:183-98 from the embryoof Ricinus communis 131. Torrey,J. G. 1965.Physiologicalbases L. Acta BoANeer[ 21:327-42 of organization and development in the 115. Simon,S. 1908. Experimentelletinter. root. In Encyclopediaof Plant Physisuchungentiber die Entstehungyon Geology 15(1):1256-1327 f~sverbindungen.Ber. Dtsc&Bot. Gex 26:364-96 132. Torrey, J. G. 1968. Hormonalcontrol of’ cytodifferentiationin agar andcell 116. Simpson,S., Torrey, J. G. 1977. Hormonalcontrol of deoxyribonucleicacid suspension cultures. In Biochemistry synthesis andprotein synthesis in pea and Physiology of Plant GrowthSubroot cortical explants. Plant Physiol. stances, ed. F. Wightman,G. Setter59:4-9 field, pp. 843-55,Ottawa:Runge Annual Reviews www.annualreviews.org/aronline Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. CONTROL OF VASCULAR DEVELOPMENT 133. Torrey, J. G. 1975. Tracheary dement formationfromsingle isolated cells in culture. Physiol. Plant 35:158-65 134. Torrey,J. G., Fosket, D. E. 1970.Cell divisionin relation to cytodifferentiation in cultured pea root segments. J. Bot. 57:1072-80 135. Torrey,J. G., Fosket,D.E., Hepler,P. K. 1971. Xylemformation: A paradigm of cytodifferentiation Ar~ ScL 59:338-52 in higherplants. 136. Tucker, S. 1959. Ontogenyof the inflorescenceandflowerin Drimyswinteri vat. dulensis. Univ. Calif. PubZBot. 30:257-336 137. Turgeon,R. 1975. Differentiation of wound vessel memberswithout DNA synthesis, mitosisor cell division. Nature 257:800-8 138. Wangermaan, E. 1967. Theeffect of the leaf on differentiation of primaryxylem in the internodeof Coleusblumeibenth. NewPhytol, 66:747-54 139. Wardiaw,C. W.1947. Experimentalinvestigations ofPhilos, the shoot apex Dryopt. eris aristatc~ Tran~ R.of Soc. Ser, 337 B 232:343-84 140.Wardlaw,C. W.1965. Theorganization of the shoot apex. In Encyclopediaof Plant Physiology15(1):966-1076 141. Wareing,P. F. 1958. Interaction be. tweenindoleaceticacid and gibbcrellic acid in eambialactivity. Nature181: 17~ ~.5 142. Wetmore, R. H., Pier, 1. P. 1963. Experimentalinductionof vasculartissues m callus of angiosperms.Am.Z Bot. 50:418-30 143. Wetmore,R. H., Sorokin, H. P. 1955. Onthe differentiation of xylem.Ant Arb. Z 36:305-17 144. Wooding,F. B. P. 1969. P-protein and microtubularsystemsin Nicotianacallus phloem.Planta 85:284-98 145. Wooding,F. B. P., Northcote, D. H. 1964. Thedevelopmentof the secondary wall of the xylemin Acer pseudoplatanu~J. Cell Sc£ 23:327-36 146.Young,B. S. 1954. Theeffects of leaf primordiaon differentiationin the stem. NewPhytol. 53:445--60 Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only. Annu. Rev. Plant. Physiol. 1979.30:313-337. Downloaded from arjournals.annualreviews.org by CAMBRIDGE UNIVERSITY on 03/27/08. For personal use only.