* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Subicular and CA1 hippocampal projections to the accessory
Aging brain wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Development of the nervous system wikipedia , lookup
Adult neurogenesis wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neuroplasticity wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Hypothalamus wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Synaptic gating wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Apical dendrite wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Subventricular zone wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Sensory cue wikipedia , lookup
Limbic system wikipedia , lookup
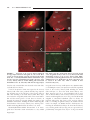
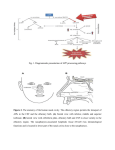
HIPPOCAMPUS 19:124–129 (2009) RAPID COMMUNICATION Subicular and CA1 Hippocampal Projections to the Accessory Olfactory Bulb C. de la Rosa-Prieto, I. Ubeda-Banon, A. Mohedano-Moriano, P. Pro-Sistiaga, D. Saiz-Sanchez, R. Insausti, and A. Martinez-Marcos* ABSTRACT: The hippocampal formation is anatomically and functionally related to the olfactory structures especially in rodents. The entorhinal cortex (EC) receives afferent projections from the main olfactory bulb; this constitutes an olfactory pathway to the hippocampus. In addition to the olfactory system, most mammals possess an accessory olfactory (or vomeronasal) system. The relationships between the hippocampal formation and the vomeronasal system are virtually unexplored. Recently, a centrifugal projection from CA1 to the accessory olfactory bulb has been identified using anterograde tracers. In the study reported herein, experiments using anterograde tracers confirm this projection, and injections of retrograde tracers show the distribution and morphology of a population of CA1 and ventral subicular neurons projecting to the accessory olfactory bulb of rats. These results extend previous descriptions of hippocampal projections to the accessory olfactory bulb by including the ventral subiculum and characterizing the morphology, neurochemistry (double labeling with somatostatin), and distribution of such neurons. These data suggest feedback hippocampal control of chemosensory stimuli in the accessory olfactory bulb. Whether this projection processes spatial information on conspecifics or is involved in learning and memory processes associated with chemical stimuli remains to be elucidated. V 2008 Wiley-Liss, Inc. C KEY WORDS: vomeronasal hippocampus; pheromone; subiculum; tract-tracing; INTRODUCTION The hippocampal formation is anatomically and functionally related to the olfactory system. The ancient view of the hippocampus as an olfactory-related structure alone, however, has been discarded for decades (Seress, 2007). Nevertheless, the hippocampal formation appears to be essential for olfactory identification (Holscher, 2003) and for the expression of odor memory representations in novel situations (Eichenbaum, 1998). Laboratorio de Neuroanatomı́a Humana, Departamento de Ciencias Médicas, Facultad de Medicina, Centro Regional de Investigaciones Biomédicas, Universidad de Castilla-La Mancha, Albacete, Spain Grant sponsor: Health Council JCCM; Grant numbers: GCS-2006_E/03; PI-2006/15; Grant sponsor: Education and Science Council JCCM; Grant number: PCC08-0064; Grant sponsor: Spanish Education and Science Ministry; Grant number: BFU2007-62290/BFI. *Correspondence to: Alino Martinez-Marcos, Dpto. CC. Médicas, Fac. Medicina, Univ. Castilla-La Mancha, Avda. Almansa 14, 02006 Albacete, Spain. E-mail: [email protected] Accepted for publication 30 July 2008 DOI 10.1002/hipo.20495 Published online 5 September 2008 in Wiley InterScience (www. interscience.wiley.com). C 2008 V WILEY-LISS, INC. Anatomically, the hippocampus receives olfactory information through several different routes. The main olfactory bulb projects directly through the medial olfactory tract to the hippocampal rudiment (LeGross Clark and Meyer, 1947; Scalia, 1966; Davis et al., 1978; Shipley and Adamek, 1984; MartinezMarcos and Halpern, 2006). Indirectly, but probably most importantly, the main olfactory bulb also projects to the entorhinal cortex (EC) (Scalia, 1966; Price, 1973; Scalia and Winans, 1975; Kosel et al., 1981; Martinez-Marcos and Halpern, 2006), which through the perforant path to the dentate gyrus (DG) provides the main input of olfactory information to the hippocampus itself (Insausti, 1993; Insausti et al., 2002; Witter, 2007). The hippocampal formation provides feedback inputs to the olfactory system by means of centrifugal projections. Historically, a projection from the temporal third of the subiculum to the anterior olfactory nucleus has been described (Swanson and Cowan, 1977). Retrogradely labeled cells were found in the ventral CA1 after large injections affecting the main and accessory olfactory bulbs and anterior olfactory nucleus (de Olmos et al., 1978). Anterograde tracing experiments in the ventral CA1 revealed a projection to the main olfactory bulb and anterior olfactory nucleus (van Groen and Wyss, 1990). The main olfactory bulb also receives centrifugal projections from the EC (Insausti et al., 1997). In addition to the main olfactory system, most mammals possess an accessory olfactory (or vomeronasal) system specialized for the detection of chemical substances such as pheromones that are involved in social interactions (Halpern and Martinez-Marcos, 2003). The relationship between the vomeronasal system and the hippocampal formation has received little attention. Vomeronasal information could reach the hippocampal formation through projections from the accessory olfactory bulb to the posteromedial cortical amygdala (Winans and Scalia, 1970; Raisman, 1972; Scalia and Winans, 1975; Davis et al., 1978; Martinez-Marcos and Halpern, 1999b; Mohedano-Moriano et al., 2007), which in turn projects to CA1, the subiculum, and the EC (Kemppainen et al., 2002). Using anterograde tracers, Cenquizca and Swanson (2007) recently described a projection, previously unknown, HIPPOCAMPAL PROJECTIONS TO THE ACCESSORY OLFACTORY BULB 125 FIGURE 1. Projections from the hippocampal formation to the accessory olfactory bulb. Coronal (A, B) and parasagittal (C, D) sections of rat brain showing anterogradely labeled fibers in the granule cell layer of the accessory olfactory bulb (D, not visible in C) after a biotinylated dextran-amine injection into CA3, ventral CA1, and ventral subiculum (A, B). Some sections were nissl-counterstained (B, C). AON, anterior olfactory nucleus; CA1, CA3, fields from the hippocampus; DG, dentate gyrus; dlo, dorsal lateral olfactory tract; FC, frontal cortex; GL, glomerular layer of the accessory olfactory bulb; Gr, granule cell layer of the accessory olfactory bulb; MOB, main olfactory bubl; M/T, mitral/tufted cell layer of the accessory olfactory bulb; PMCo, posteromedial cortical amygdaloid nucleus; VS, ventral subiculum. Calibration bar for: A–C 400 lm; D 40 lm. from the ventral field of CA1 to the granule cell layer of the accessory olfactory bulb. Earlier studies on centrifugal projections to the accessory olfactory bulb using retrograde tracers, however, had failed to reveal such projections (Raisman, 1972; Broadwell and Jacobowitz, 1976; Davis et al., 1978; Barber, 1982; Martinez-Marcos and Halpern, 1999a). Only large injections also affecting the main olfactory bulb and anterior olfactory nucleus yielded labeling in the ventral CA1 (de Olmos et al., 1978). This work aims to identify the origin of centrifugal afferents from the CA1 and subiculum to the accessory olfactory bulb in order to further explore the connectivity between these systems. These data could eventually increase our understanding of the influence of the hippocampal formation on the processing of chemical cues. Twenty-three adult female Sprague-Dawley rats were used in this study. Throughout the study, the guidelines of the European Community on Welfare of Research Animals (directive 86/609/EEC) and the Ethical Committee for Animal Research at the University of Castilla-La Mancha were followed. Animals were injected intraperitoneally with a combined dose of ketamine hydrochloride (Ketolar, Parke-Davis, Madrid, 75 mg/kg) and xylazine (Xilagesic, Barcelona, 10 mg/kg). Iontophoretic injections of dextran-amines conjugated to biotin (BDA) or tetramethylrhodamine (RDA, Molecular Probes, Eugene, OR) or FluoroGold (FG, Biotium, Hayward, CA) were placed into ventral CA1/ventral subiculum or the accessory olfactory bulb. Five days later, the animals were anesthetized (as above) and perfused with 4% paraformaldehyde. Sagittal (olfactory bulbs) or coronal sections were obtained using a freezing microtome. Biotinylated dextran-amine (BDA) was revealed using the ABC kit (Vector Laboratories, Burlingame, CA). Some sections of RDA experiments were reacted for immunofluorescence against somatostatin (1:1,000, goat antisomatostatin D-20, Santa Cruz; 1:200 chicken anti-goat Alexa 488, Molecular Probes) to analyze the possible correspondence with the two types of subicular neurons (Witter and Amaral, 2004). For further details of the protocols see related articles (Pro-Sistiaga et al., 2007; Ubeda-Banon et al., 2007, 2008). A total of 23 injections of different tracers were performed at different locations. Five injections of BDA were aimed at the ventral portion of CA1 and/or the ventral subiculum; all yielded comparable results. Case B10207 is described as representative. The injection site was small, involving an area between ventral CA1 and the ventral subiculum and a portion of CA3 (Figs. 1A,B). It is important to note that the posteromedial cortical amygdala was completely unaffected by the injection site. Fine beaded fibers were observed in the granule cell layer of the accessory olfactory bulb (Fig. 1C-not observable at this magnification-, arrowheads in D). Abundant anterograde labeling was observed in the anterior olfactory nuHippocampus 126 DE LA ROSA-PRIETO ET AL. FIGURE 2. Projections to the accessory olfactory bulb from the hippocampal formation. Parasagittal (A) and coronal (B–F) sections of rat brain showing retrograde and anterograde labeling (B–F) after a tetramethylrhodamine-labeled dextran-amine injection into deep layers (mitral/tufted and granule cell layers) of the accessory olfactory bulb (A). Double labeling with somatostatin immunofluorescence (F). CA1, CA3, fields from the hippocampus; DG, dentate gyrus; dlo, dorsal lateral olfactory tract; FC, frontal cortex; Gr, granule cell layer of the accessory olfactory bulb; M/T, mitral/tufted cell layer of the accessory olfactory bulb; PMCo, posteromedial cortical amygdaloid nucleus; VS, ventral subiculum. Calibration bar for: A, B 400 lm; C–F: 80 lm. [Color figure can be viewed in the online issue, which is available at www. interscience.wiley.com.] cleus, but only scattered fibers were observed in the main olfactory bulb (data not shown). A total of six injections of FG were targeted to the accessory olfactory bulb. This tracer produced relatively large injection sites involving most of the cell layers of the accessory olfactory bulb often extending to the main olfactory bulb. These injections resulted in retrogradely labeled cells in the ventral subiculum and the ventral portion of CA1 (data not shown). As expected, the pattern of retrograde labeling was similar to that described in the literature, including the anterior medial amygdala, the bed nucleus of the accessory olfactory tract, and the posteromedial cortical amygdaloid nucleus (de Olmos et al., 1978). Tetramethylrhodamine-labeled dextran-amine is an anterograde tracer that under certain circumstances is a very reliable retrograde tracer from very small injection sites (Martinez-Marcos and Halpern, 1999a). Six injections involved the superficial layers of the accessory olfactory bulb including the mitral/ tufted cell layer, i.e., above the dorsal lateral olfactory tract. These injections gave rise to retrograde-labeled cells in areas such as the bed nucleus of the accessory olfactory tract and the anterior medial amygdala, but not in the posteromedial medial amygdala or CA1/ventral subiculum (data not shown). Six injections affected the deep layers of the accessory olfactory bulb, i.e., including the granule cell layer; all yielded consistent results. As example, case R6807 showed a small injection site involving the mitral/tufted cell layer, the dorsal lateral olfactory tract, and the granule cell layer (Fig. 2A). As expected from a predominantly anterograde tracer, layer I of the posteromedial Hippocampus HIPPOCAMPAL PROJECTIONS TO THE ACCESSORY OLFACTORY BULB 127 FIGURE 3. Mapping of distribution of labeled cells. Schematic sections of the brain illustrating one example of the distribution of retrogradely labeled cells after a rhodamine-labeled dextran-amine injection into the granule cell layer of the accessory olfactory bulb. Numbers indicate the distance from Bregma according to Paxinos and Watson (2005). The Insausti et al.’ (1997) nomenclature for the entorhinal cortex has been adopted. Abbreviations: APir, amygdalo-piriform transition area; CA1, CA2, CA3, fields from the hippocampus; DG, dentate gyrus; DIE, dorsal intermediate entorhinal field; DLE, dorsal lateral entorhinal field; Pir, piriform cortex; PMCo, posteromedial cortical amygdaloid nucleus; rf, rhinal fissure; VS, ventral subiculum. cortical amygdaloid nucleus showed numerous terminal fibers (Fig. 2B). This injection gave rise to retrograde-labeled cells in the bed nucleus of the accessory olfactory tract and anterior medial amygdala (data not shown) as well as in the posteromedial cortical amygdaloid nucleus, ventral CA1, and ventral subiculum (Fig. 2B). In ventral CA1, cells were deeply located in the stratum oriens and showed a main ascending dendrite (Fig. 2C). This pattern was similar in the ventral subiculum (Fig. 2D), although some cells were superficially placed and showed apical dendrites and proximal branches near the cell somata (arrows in Fig. 2E). Labeled cells did not coexpress somatostatin (Fig. 2F). Topographically, these cells were restricted to the ventral CA1 and ventral subiculum at mid rostrocaudal levels from 24.80 to 25.88 mm from bregma according to Paxinos and Watson (2005) (Figs. 3A–C). Scattered cells were observed in the piriform and entorhinal cortices (Fig. 3). Collectively, these results demonstrate only recently and partially known feedback projection from the ventral area of CA1 of the hippocampus and from ventral subiculum to the granule cell layer of the accessory olfactory bulb. This projection was shown to arise from the ventral field of CA1 using anterograde tracing (Cenquizca and Swanson, 2007). Our results are interesting and novel for four reasons: (1) we have retrogradely confirmed such a projection for the first time; (2) we have demonstrated not only the projection of CA1 but also that of the ventral subiculum to the accessory olfactory bulb; (3) considering the number of retrograde-labeled cells, these results show that the hippocampus may have an influence on the vomeronasal system, which has been underestimated previously; (4) the data on cell position (deep and superficial), dendritic morphology (narrow and wide), and double labeling with somatostatin (no coexpression) suggest that labeled cells could correspond to the two cell types described in the subiculum (Harris et al., 2001): intrinsically bursting cells tend to be deeply located, have a narrow dendritic tree, and preferentially express somatostatin; whereas regular spiking neurons tend to be located superficially, have a wider dendritic tree, and preferentially express NADPH diaphorase/nitric oxide synthetase (NOS) (Witter and Amaral, 2004). Therefore, according to our data on position and cell morphology, and although we have not found double-labeled cells with somatostatin and this is unconclusive, we believe that both cell types could be projecting to the accessory olfactory bulb although eletrophysiological data would be necessary to asses this point. Prior to discussing these results, it is necessary to include some technical considerations. Injections in the accessory olfactory bulb involved the dorsal portion of the lateral olfactory tract and the possibility arises that axons traveling to the main olfactory bulb were affected by the injection site. Regarding this possibility, it is necessary to distinguish the labeling in the ventral CA1 and ventral subiculum from the labeling in the piriform and entorhinal cortices. The labeling in CA1 and ventral subiculum likely corresponds to axons ending in the accessory olfactory bulb based in three reasons: (1) the retrograde labeling in CA1 and ventral subiculum was abundant after injections in the accessory olfactory bulb (Figs. 2 and 3), but absent after injections in the main olfactory bulb (Insausti et al., 1997; unpublished results); (2) the anterograde labeling in the accessory olfactory bulb was terminal-like (Cenquizca and Swanson, 2007) (Fig. 1D) after injections in CA1 and ventral subiculum (Figs. 1A,B); (3) according to the literature, the ventral CA1 and ventral subiculum projects to the main olfactory bulb (van Groen and Wyss, 1990), but this projection is Hippocampus 128 DE LA ROSA-PRIETO ET AL. much reduced when compared with the projections to the anterior olfactory nucleus and accessory olfactory bulb (Cenquizca and Swanson, 2007) (this report). On the other hand, the labeling in the piriform and entorhinal cortices after injections in the accessory olfactory bulb is likely due to involvement of axons in the lateral olfactory tract traveling to the main olfactory bulb based in two reasons: (1) only scattered cells were seen in the piriform and entorhinal cortices after injections in the accessory olfactory bulb (Figs. 2 and 3); (2) this pattern of labeling in the piriform and entorhinal cortices is comparable, although more abundant, after injections in the main olfactory bulb (Insausti et al., 1997; unpublished results). Therefore, we are confident that regrogradely labeled cells in the ventral CA1 and ventral subiculum originate from axons ending in the granule cell layer of the accessory olfactory bulb. It is interesting to speculate why the projection from the ventral CA1 and ventral subiculum to the accessory olfactory bulb has not been revealed by previous studies of the afferent connections. Studies using lesion-degeneration methods (Raisman, 1972), autoradiography with tritiated amino acids (Barber and Field, 1975; Davis et al., 1978; Barber, 1982), tracers such as horseradish peroxidase (HRP) (Broadwell and Jacobowitz, 1976; Davis et al., 1978) or dextran-amines (MartinezMarcos and Halpern, 1999a) have not shown retrograde labeling in CA1 or subiculum. Only injections of HRP affecting the main and accessory olfactory bulbs and anterior olfactory nucleus yielded retrograde-labeled cells in the ventral CA1 (de Olmos et al., 1978), although authors were unsure of this projection due to the large sizes of their injections. The reason could be methodological and/or due to species differences. Sensitive retrograde tracers such as FG gave rise to retrogradely labeled cells in ventral CA1 and ventral subiculum, but injections of RDA only produced such retrograde labeling when the tracer was greatly concentrated and affected the granule cell layer (present results). It is therefore likely that these projections parallel the centrifugal pathway from the posteromedial cortical amygdaloid nucleus through the stria terminalis (Raisman, 1972; Barber, 1982). In fact, the accessory olfactory bulb receives differential laminar projections: one bundle of fibers from the posteromedial cortical amgydaloid nucleus traveling through the stria terminalis that reaches the internal granule cell layer, and a second bundle of fibers from the medial amgydaloid nucleus and bed nucleus of the accessory olfactory tract terminating in the internal plexiform layer and probably running in the accessory olfactory tract (Barber, 1982). These results are in line with a third pathway from the hippocampal formation through the septal complex and taenia tecta, as already described (van Groen and Wyss, 1990; Cenquizca and Swanson, 2007), that would reach exclusively the granule cell layer of the accessory olfactory bulb. Therefore, the absence of labeling in previous studies could be due to the sensitivity of the method used including the precise placement of the injection site (Raisman, 1972; Barber and Field, 1975; Broadwell and Jacobowitz, 1976; Davis et al., 1978; de Olmos et al., 1978; Barber, 1982) or to the fact that such projections are absent in marsupials mammals such as the short-tailed opossum (Martinez-Marcos and Halpern, 1999a). Hippocampus Regarding anterograde tracing experiments aimed at the hippocampus, injections of tritiated amino acids into the ventral subiculum yielded labeling in the anterior olfactory nucleus (Swanson and Cowan, 1977); whereas injections of Phaseolus vulgaris-leucoagglutinin (PHAL) in the ventral CA1 confirmed the projection to the anterior olfactory nucleus and revealed a projection to the olfactory bulb (it is assumed to the main olfactory bulb) (van Groen and Wyss, 1990). Only recently, similar experiments with injections of PHAL in the ventral CA1 has specifically described the projection to the granule cell layer of the accessory olfactory bulb (Cenquizca and Swanson, 2007). Although the ancient view of the hippocampus as a mainly olfactory structure is not longer accepted (Seress, 2007), there is a clear anatomical and functional relationship between the hippocampus and the olfactory system. Anatomically, the main olfactory bulb projects to the EC (Price, 1973; Scalia and Winans, 1975; Davis et al., 1978; Shipley et al., 2004), which in turn constitutes one of the main sources of afferents to the hippocampus (Witter and Amaral, 2004; Witter, 2007). Functionally, a number of lines of evidence support the participation of the hippocampal formation in olfactory learning and memory (Eichenbaum, 1998; Truchet et al., 2002; Roman et al., 2004). However, data on the relationship between the hippocampal formation and the vomeronasal system are sparse (Kemppainen et al., 2002; Cenquizca and Swanson, 2007). The data available, however, allow proposing circuits between the hippocampal formation and the vomeronasal system and hypothesizing about their functional significance. A number of connections between the amygdaloid complex, including the vomeronasal amygdala, and the hippocampal formation have been described (Pitkanen et al., 2000). For example, the posterior cortical nucleus of the amygdala receives vomeronasal information from the accessory olfactory bulb (Winans and Scalia, 1970; Scalia and Winans, 1975; Mohedano-Moriano et al., 2007) and projects to the EC, ventral subiculum, and ventral CA1(Kemppainen et al., 2002). The ventral subiculum and ventral CA1 project to the accessory olfactory bulb (Cenquizca and Swanson, 2007) (present results). Therefore, these connections would constitute a feedback loop from the accessory olfactory bulb to the posteromedial cortical amygdaloid nucleus, from there to the ventral subiculum and ventral CA1 and back to the accessory olfactory bulb. This circuit could participate in recognition and memory formation of pheromones, in a manner similar to that of recognition and memory formation of odors (Eichenbaum, 1998; Roman et al., 2004). Alternatively, the projection from the ventral CA1 and ventral subiculum to the accessory olfactory bulb could relay spatial information on the location of conspecific chemical signals from the cognitive map elaborated by place cells (O’Keefe, 1979; Brun et al., 2002). Acknowledgments The authors thank members of LNH and Dr. Garcı́a Olmo for her help with the animals. The assistance of International Science Editing in revising the English version of the manu- HIPPOCAMPAL PROJECTIONS TO THE ACCESSORY OLFACTORY BULB script is also acknowledged. CRP is supported by a predoctoral fellowship associated with Grant PCC08–0064. REFERENCES Barber PC. 1982. Adjacent laminar terminations of two centrifugal afferent pathways to the accessory olfactory bulb in the mouse. Brain Res 245:215–221. Barber PC, Field PM. 1975. Autoradiographic demonstration of afferent connections of the accessory olfactory bulb in the mouse. Brain Res 85:201–203. Broadwell RD, Jacobowitz DM. 1976. Olfactory relationships of the telencephalon and diencephalon in the rabbit. III. The ipsilateral centrifugal fibers to the olfactory bulbar and retrobulbar formations. J Comp Neurol 170:321–345. Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. 2002. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science 296:2243–2246. Cenquizca LA, Swanson LW. 2007. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev 56:1–26. Davis BJ, Macrides F, Youngs WM, Schneider SP, Rosene DL. 1978. Efferents and centrifugal afferents of the main and accessory olfactory bulbs in the hamster. Brain Res Bull 3:59–72. de Olmos J, Hardy H, Heimer L. 1978. The afferent connections of the main and the accessory olfactory bulb formations in the rat: An experimental HRP-study. J Comp Neurol 181:213–244. Eichenbaum H. 1998. Using olfaction to study memory. Ann N Y Acad Sci 855:657–669. Halpern M, Martinez-Marcos A. 2003. Structure and function of the vomeronasal system: An update. Prog Neurobiol 70:245–318. Harris E, Witter MP, Weinstein G, Stewart M. 2001. Intrinsic connectivity of the rat subiculum. I. Dendritic morphology and patterns of axonal arborization by pyramidal neurons. J Comp Neurol 435:490–505. Holscher C. 2003. Time, space and hippocampal functions. Rev Neurosci 14:253–284. Insausti R. 1993. Comparative anatomy of the entorhinal cortex and hippocampus in mammals. Hippocampus 3 Spec No: 19–26. Insausti R, Herrero MT, Witter MP. 1997. Entorhinal cortex of the rat: Cytoarchitectonic subdivisions and the origin and distribution of cortical efferents. Hippocampus 7:146–183. Insausti R, Marcos P, Arroyo-Jimenez MM, Blaizot X, Martinez-Marcos A. 2002. Comparative aspects of the olfactory portion of the entorhinal cortex and its projection to the hippocampus in rodents, nonhuman primates, and the human brain. Brain Res Bull 57: 557–560. Kemppainen S, Jolkkonen E, Pitkanen A. 2002. Projections from the posterior cortical nucleus of the amygdala to the hippocampal formation and parahippocampal region in rat. Hippocampus 12:735–755. Kosel KC, Van Hoesen GW, West JR. 1981. Olfactory bulb projections to the parahippocampal area of the rat. J Comp Neurol 198: 467–482. LeGross Clark WE, Meyer M. 1947. The terminal connections of the olfactory tract in the rabbit. Brain 70:304–328. Martinez-Marcos A, Halpern M. 1999a. Differential centrifugal afferents to the anterior and posterior accessory olfactory bulb. Neuroreport 10:2011–2015. Martinez-Marcos A, Halpern M. 1999b. Differential projections from the anterior and posterior divisions of the accessory olfactory bulb to the medial amygdala in the opossum, Monodelphis domestica. Eur J Neurosci 11:3789–3799. Martinez-Marcos A, Halpern M. 2006. Efferent connections of the main olfactory bulb in the opossum (Monodelphis domestica): A characterization of the olfactory entorhinal cortex in a marsupial. Neurosci Lett 395:51–56. 129 Mohedano-Moriano A, Pro-Sistiaga P, Ubeda-Banon I, Crespo C, Insausti R, Martinez-Marcos A. 2007. Segregated pathways to the vomeronasal amygdala: Differential projections from the anterior and posterior divisions of the accessory olfactory bulb. Eur J Neurosci 25:2065–2080. O’Keefe J. 1979. A review of the hippocampal place cells. Prog Neurobiol 13:419–439. Paxinos G, Watson C. 2005. The Rat Brain in Stereotaxic Coordinates. San Diego: Elsevier. Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. 2000. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci 911:369–391. Price JL. 1973. An autoradiographic study of complementary laminar patterns of termination of afferent fibers to the olfactory cortex. J Comp Neurol 150:87–108. Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Del Mar Arroyo-Jimenez M, Marcos P, Artacho-Perula E, Crespo C, Insausti R, Martinez-Marcos A. 2007. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J Comp Neurol 504:346–362. Raisman G. 1972. An experimental study of the projection of the amygdala to the accessory olfactory bulb and its relationship to the concept of a dual olfactory system. Exp Brain Res 14:395–408. Roman FS, Truchet B, Chaillan FA, Marchetti E, Soumireu-Mourat B. 2004. Olfactory associative discrimination: A model for studying modifications of synaptic efficacy in neuronal networks supporting long-term memory. Rev Neurosci 15:1–17. Scalia F. 1966. Some olfactory pathways in the rabbit brain. J Comp Neurol 126:285–310. Scalia F, Winans SS. 1975. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol 161:31–55. Seress L. 2007. Comparative anatomy of the hippocampal dentate gyrus in adult and developing rodents, non-human primates and humans. Prog Brain Res 163:23–41. Shipley MT, Adamek GD. 1984. The connections of the mouse olfactory bulb: A study using orthograde and retrograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. Brain Res Bull 12:669–688. Shipley MT, Ennis M, Puche AC. 2004. Olfactory system. In: Paxinos G, editor. The Rat Nervous System, 3rd ed. San Diego: Elsevier Academic Press. pp 923–964. Swanson LW, Cowan WM. 1977. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol 172:49–84. Truchet B, Chaillan FA, Soumireu-Mourat B, Roman FS. 2002. Learning and memory of cue-reward association meaning by modifications of synaptic efficacy in dentate gyrus and piriform cortex. Hippocampus 12:600–608. Ubeda-Banon I, Novejarque A, Mohedano-Moriano A, Pro-Sistiaga P, de la Rosa-Prieto C, Insausti R, Martinez-Garcia F, Lanuza E, Martinez-Marcos A. 2007. Projections from the posterolateral olfactory amygdala to the ventral striatum: Neural basis for reinforcing properties of chemical stimuli. BMC Neurosci 8:103. Ubeda-Banon I, Novejarque A, Mohedano-Moriano A, Pro-Sistiaga P, Insausti R, Martinez-Garcia F, Lanuza E, Martinez-Marcos A. 2008. Vomeronasal inputs to the rodent ventral striatum. Brain Res Bull 75:467–473. van Groen T, Wyss JM. 1990. Extrinsic projections from area CA1 of the rat hippocampus: Olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J Comp Neurol 302:515–528. Winans SS, Scalia F. 1970. Amygdaloid nucleus: New afferent input from the vomeronasal organ. Science 170:330–332. Witter MP. 2007. The perforant path: Projections from the entorhinal cortex to the dentate gyrus. Prog Brain Res 163:43–61. Witter MP, Amaral DG. 2004. Hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. San Diego: Elsevier. pp 635–704. Hippocampus