* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download biochemistry-16
Basal metabolic rate wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Genetic code wikipedia , lookup
Photosynthesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Drug discovery wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Proteolysis wikipedia , lookup
Biosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
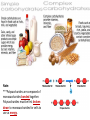
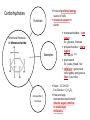
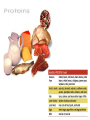
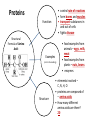
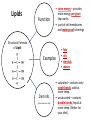
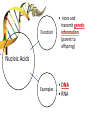
You are what you eat!? Chemistry – study of what substances are made of and how they change and combine. • Each different kind of atom is a different element. Examples of elements: C – Carbon H – Hydrogen O – Oxygen N – Nitrogen • Two or more elements combine to make a compound. Examples of compounds – H2O, CO2, HCl, NaCl • Compounds are classified into 2 groups: 1. Inorganic Compounds – come from nonliving substances (In = not Organic = living) Ex: H2O is the universal solvent because of its bent molecular shape and polarity. Water dissolves other polar compounds by pulling them apart like a magnet separates metals. Positive and negative ends like the poles of a magnet. *70-80% of your body is water 2. Organic Compounds – come from living substances Biochemistry – study of the chemistry of living organisms • All organic compounds will have the element carbon in them Exception: CO2 is not organic (CO2 is not composed of living substances.) • Organic compounds are usually complex compounds with many atoms in their structure. Ex: Glucose – C6H12O6 • Four kinds of organic compounds: 1. Carbohydrates 2. Proteins 3. Lipids 4. Nucleic acids – DNA and RNA Note: ***Polysaccharides are composed of monosaccharides bonded together. Polysaccharides must be first broken down to monosaccharides for cells to use as energy. Carbohydrates Function • first and preferred energy source of cells • structural support in plants Structural Formula of Monosaccharide: Examples Composition / Structure • monosaccharides – (one sugar) Ex: glucose, fructose • polysaccharides – (many sugars) Ex: starch *** • plant starch Ex: pasta, bread, rice • cellulose – gives plant cells rigidity and gives us fiber in our diet • Ratio – 1C:2H:1O Ex: Glucose – C6H12O6 • How are large macromolecules formed? Smaller sugars combine to make larger molecules. • control rate of reactions • form bones and muscles • transport substances in and out of cells • fights disease Proteins Function Structural Formula of Amino Acid: Examples (not in the reading) Structure • food examples from animals – eggs, milk, meat • food examples from plants – nuts, beans • enzymes • elements involved – C, N, H, O • proteins are composed of – amino acids • How many different amino acids are there? 20 General Structure of Amino Acid Alanine Serine Protein Amino Acids Lipids Function • store energy – provides more energy per gram than carbs • part of cell membranes and waterproof coverings Structural Formula of Lipid: Examples Diet info (to be covered in class) • • • • fats oils steroids waxes • saturated – contains only single bonds, solid at room temp. • unsaturated – contains double bonds, liquid at room temp. (Better for your diet.) Function • store and transmit genetic information (parent to offspring) Examples • DNA • RNA Nucleic Acids Enzymes • Catalyst—substance that accelerates the rate of a chemical reaction Ex: hydrogen peroxide being broken down into water and oxygen H2O2 H2O + O2 add catalase for FASTER REACTION! • • • • • • • Enzyme – a kind of catalyst found only in living things Enzymes are proteins Enzymes change only the speed of the reaction Enzymes are never used up in a reaction, so they can be used over and over Enzymes are specific for the reaction they catalyze Ex: Saltines and amylase Starch Sugar Enzymes allow digestion to occur faster; otherwise the hamburger you ate last week might still be in your stomach! By using enzymes to break chemical bonds in food molecules, organisms release energy for life processes