* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Gene expression analysis to evaluate the effect of p38 specific
Point mutation wikipedia , lookup
X-inactivation wikipedia , lookup
Gene therapy wikipedia , lookup
Genome evolution wikipedia , lookup
RNA interference wikipedia , lookup
History of genetic engineering wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Genomic imprinting wikipedia , lookup
Gene expression programming wikipedia , lookup
Ridge (biology) wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Microevolution wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Oncogenomics wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Genome (book) wikipedia , lookup
Minimal genome wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Designer baby wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Gene expression profiling wikipedia , lookup
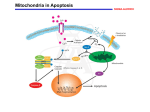
Gene expression analysis to evaluate the effect of p38 specific inhibitor SB203580 on SEB-induced apoptosis related pathways By: Lisa Hesse, Advisor—Dr. Chanaka Mendis Abstract Staphylococcal enterotoxin B (SEB) is a bacterial toxin that has been thoroughly investigated. However, little is known about the cascades of signaling events that explain its patho-mechanism. This research involves the pathogenic nature of SEB, which can cause death in human peripheral blood mononuclear cells (PBMC) by inducing multiple signal transduction pathways. Inhibiting crucial pathway inter-connector (p38) may alter unwanted, SEB-induced, cellular activities. This study focuses on obstructing signaling pathways using inhibitor SB203580 and analyzing alterations to known expression patterns of genes associated with apoptosis [the Caspase genes and Heparanse precursor (HEP)]. As our experimental design specifically targets p38, we believe our target is unique and has the ability to sustain longer lasting inhibitory effects. We are also confident that inhibiting such a component will have a maximum effect on a cell module, terminating the “leaking effect,” which may benefit other experimental modules focused on disease prevention and rapid diagnostics. Introduction Staphylococcal enterotoxin B (SEB) is a toxin secreted by the bacterium Staphylococcus aureus and causes a person to have symptoms of food poisoning. These flu-like symptoms, if overlooked for a prolonged period of time, can cause a lot of harm to a person, and possibly lead to death. S. aureus is found in improperly refrigerated meats and dairy products and will survive unless food is cooked thoroughly. Once in the body, the bacterium releases the SEB toxin, triggering a multitude of cell signaling pathways leading up to the flu-like symptoms associated with food poisoning. The genes activated in these cell pathways have been sequenced and are well known; however, a way to inhibit the activation of such genes has yet to be perfected. It is known that the structure of SEB contains two units of the amino acid cysteine in the middle of the whole protein forming a disulphide bridge when the toxin is mature with an intervening variable loop (Jett 1092). SEB is different form the other staphylococcal enterotoxins (SEs) such as SEA, C1, C2, C3, D, E, G, H, I, and J due to its affinity for different cell types as well as for the binding sites of the class II major histocompatibility complex (MHC II) and T-cell receptors (Jett 1093). It has been found that multiple conserved residues on the smaller domain structure of the toxin as well as in the N- and C-terminal regions allow SEB to bind to the β subunit of MHC II (Jett 1093). The binding of SEB to MHC II activates polyclonal T lymphocytes, the cells of the immune system that are responsible for killing viral infected cells (Blazes). In an attempt to determine the exact pathways SEB induces within a cell, the various gene expressions of genes known to be associated with apoptosis will be analyzed and quantified when compared to the expression of a housekeeping gene. This project focuses in on the p38 inter connector, which is known to have an influence on the apoptosis pathway induced by the SEB toxin. By introducing an inhibitor to this interconnector, SB203580, the effect of blocking the pathway may allow for a complete specific gene pathway to be determined. Once the pathway is mapped, the information has the potential to provide medicinal breakthroughs in the fields of cell death and aging. Methods QUANTIFICATION OF RNA AND PROTEIN SAMPLES RNA and protein samples were isolated from three types of human PBMCs (Peripheral Blood Mononuclear Cells): 1) control (not treated with anything at 2 and 6 hours), 2) toxin exposed cells with inhibitor for 2 and 6 hrs, and 3) toxin exposed cells without the inhibitor at 2 and 6 hours (WRAIR, Silver Spring, MD). These samples were then quantitated using a UV-VIS spectrometer (PerkinElmer Lambda 900 UV/VIS/NIR Spectrometer). REVERSE TRANSCRIPTION (RT-PCR) RT was done using an iScript™ cDNA Synthesis Kit (BIO-RAD) in a Thermocycler (Eppendorf Mastercyler Personal). POLYMERASE CHAIN REACTION (PCR) PCR was carried out using primers of genes of interest designed through various kinds of primerdesign software in a Thermocycler (Eppendorf Mastercycler Personal) using a PCR master mix kit (Roche Diagnostics, Indianapolis, IN) VISUALIZATION & QUANTIFICATION All PCR samples were analyzed on 1% agarose gels using syber green for fluorescence and were visualized through an in house imaging apparatus and quantitated by Image J analysis. ELISA All samples were either run using the Caspase-3, Caspase-8, or Caspase-10 Colorimetric Assay Kits from BioVision (Mountain View, CA). The type of instrument used was a Microplate Autoreader EL311 Bio-Tek Instrument. Results and Discussion Previous research has discovered that SOD genes regulate caspase-1 activation through reversible oxidation and glutathionylation of cysteine residues (Meissner). It has also been discovered that USP has been shown to play a large part in caspase regulation and cell survival through multiple ubiquitin pathway proteins and their effect on targeted degradation via the ubiquitin-proteasome pathway, specifically with its effect on the gene IAP (Steller). With this knowledge, a set of genes was selected in order to attempt to piece a signaling pathway together. From the results obtained thus far, Caspase 1, 6, 7, 9, 10, HEP, SOD, USP, STK17A, and REQ were all up-regulated at both the 2 and 6 hour exposures by SEB (Table 1). The results also showed that the p38 inhibitor SB203580 was able to alter the expression of all SEB induced apoptosis related genes (Table 1). From these results, it is clear that SEB affects the intrinsic apoptosis pathway. The intrinsic apoptosis pathway consists of the induction of Caspase 9 followed by the induction of Caspase 3, 6, and 7. On the other hand, the extrinsic apoptosis pathway consists of the induction of Caspase 8 followed by the induction of Caspase 10, 3, 6, and 7. Due to the values of 16.9 ± 2.0 (SEB 2 hr) and 10.4 ± 0.4 (SEB 6 hr) it can be said that SEB affects the apoptosis pathway due to the up-regulation of Caspase 9. In conjunction with the testing of RNA samples with the genes of interest, protein testing has begun with Caspase 3 and Caspase 8 (Table 2). As done with the RNA data, calculations are performed to normalize the absorbance readings and from this data no conclusive results as to the apoptosis pathway affected can be obtained due to the fact that the protein of Caspase 9 has not been tested yet. Gene Time Counts 2 hr Time Counts 6 hr 24 hr p38 (inhibitor) Caspase 1 8.8 ± 0.1 6.2 ± 0.1 1.5 ± 0.4 Caspase 6 4.0 ± 0.4 6.3 ± 0.3 0.9 ± 0.5 Caspase 7 5.8 ± 0.4 5.5 ± 0.2 1.4 ± 0.6 Caspase 9 16.9 ± 2.0 10.4 ± 0.4 4.5 ± 3.3 Caspase 10 6.8 ± 0.4 8.5 ± 0.3 1.5 ± 0.4 HEP 11.2 ± 0.0 11.5 ± 0.3 2.1 ± 0.1 SOD 7.0 ± 0.0 7.6 ± 0.3 1.5 ± 0.1 USP 8.1 ± 0.2 6.8 ± 0.0 1.6 ± 0.3 STK17A 13.0 ± 0.1 13.0 ± 0.1 3.2 ± 0.2 REQ 4.8 ± 0.0 8.1 ± 0.0 1.8 ± 0.2 Table 1: These calculated regulation values were obtained using the absorbance value of the gene of interest and the housekeeping gene. Gene Caspase 3 Caspase 3 Caspase 3 Caspase 3 Caspase 3 Caspase 3 Caspase 3 Caspase 8 Caspase 8 Caspase 8 Caspase 8 Caspase 8 Caspase 8 Caspase 8 Sample SEB-SP 2H A 5-LPS SP A SEB 12 H B 5-LPS PD B LPS 2H B SEB-SB 2H A LPS 2-C A SEB-SP 2H A 5-LPS SP A SEB 12 H B 5-LPS PD B LPS 2H B SEB-SB 2H A LPS 2-C A Calculated Values: #/C Absorbance at 405 nm 1.679 0.047 1.393 0.039 1.821 0.051 0.893 0.025 1.893 0.053 2.250 0.063 1.000 0.028 2.706 0.046 1.529 0.026 1.941 0.033 1.176 0.020 1.765 0.030 1.588 0.027 1.000 0.017 Table 2: Elisa absorbance readings which were prepared using Caspase 3 and 8 kits. Conclusion and Future Plans While there are still many genes to be tested in potential apoptosis pathways of human peripheral blood mononuclear cells, this project yielded promising results. Since a lot of the genes tested have been effectively down-regulated by the p38 pathway inhibitor SB203580, the potential for mapping out the apoptotic pathway of the SEB toxin is growing with each additional gene studied. Currently, reproducible results are in the process of being obtained, so that observations can be confirmed, along with other genes that are associated with apoptosis: GRIM 19, NGFRAP1, MAPKAPK5, PDCD4, MIHC, Caspase 3, and Caspase 8. As the data pool grows, the apoptotic pathway can be traced and confirmed, thus being a vital study to the hopes of inhibiting cell death in affected cells. References Blazes, David L., Lawler, James V., Lazarus, & Angeline A. “When Biotoxins are Tools of Terror”. Postgraduate Medicine, Vol. 112:2. Section Symposium: Third of Three Articles on Bioterrorism Jett, Marti, Ionin, B., Das, R., & Neill, R. “The Staphylococcal Enterotoxins”. Walter Reed Army Institute of Research, Silver Spring, MD, USA. 2001 Meissner, Felix, K. Molawi, A Zychlinsky. “Superoxide dismutase 1 regulates caspase-1 and endotoxic shock” Nature Immunology, Vol. 9:8 pp: 866-872. August 2008. Mendis, Chanaka, Campbell, K., Das, R., Yang D., & Jett, M. “Effect of 5-Lipoxygenase inhibitor MK591 on early molecular and signaling events induced by staphylococcal enterotoxin B (SEB) in human peripheral blood mononuclear cells (PBMCs)” FEBS Journal, May 2008 Steller, H. “Regulation of apoptosis in Drosphilia” Cell Death and Differentiation, (15) 11321138. 2008