* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download So, you want to know about siderophore synthesis
Enzyme inhibitor wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Catalytic triad wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Butyric acid wikipedia , lookup
Citric acid cycle wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Proteolysis wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Siderophore wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Oligonucleotide synthesis wikipedia , lookup
Biosynthesis of doxorubicin wikipedia , lookup
Amino acid synthesis wikipedia , lookup
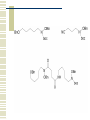
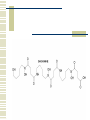
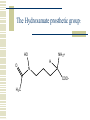
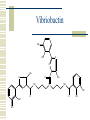
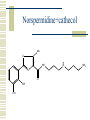
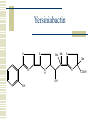
So, you want to know about siderophore synthesis Presented by: Steven Backues Brooks Maki and Donnie Berkholz “The Invisible Man” Hydroxamic Acid Groups N-Alkylation of O-substituted Hydroxamic acid. Formation of an oxime from an aldehyde and a hydroxylamine. Followed by reduction and acylation These derivatives allowed synthesis of several siderophores and their analogues Alternative Methods Oxidation of Lysine and OrnithineWith DMD (dimethyldioxarine) Conversion of primary amines to imines, with oxidation of imines to oxaziridines. Hydrolysis leads to hydroxylamines. D-Ferrichrome synthesized by this method Danoxamine Composed of a linear series of three hydroxamic acids. Composed of two major groups linked by succinic acid. Biosynthesis of Siderophores How it’s really done. Hydroxamate Siderophores Step 1: Ornithine N5Oxygenase The formation of the N-O bond is the first committed step in hydroxamate synthesis Ornithine, an amino acid used in the urea cycle, is reacted with O2 and NADPH to give an N-O bond at the end of its side chain. Step 2: 5N Transacylase The nitrogen modified in this way is additionally attached to an acyl group carried by coenzyme A This completes the hydroxamate prosthetic group The Hydroxamate prosthetic group: HO O NH 3+ H N COO- H3C Step 3: Non-ribosomal Peptide Synthetase This synthetase is a large complex with many subdomains, including an adenylation domain, a thiolation domain, and a condensation domain. Adenylation First, the hydroxamate is is activated by addition of an adenylate group at its C terminus The source of the adenylate group is ATP, and the reaction occurs with production of pyrophosphate Thiolation The hydroxamate group is then transferred to the enzyme through the formation of a thioester linkage with displacement of the adenylate group. Condensation Finally, the hydroxamate group is attached to another molecule (perhaps another hydroxamate group, or else a growing chain) by the nucleophilic attack of an OH or NH from the chain on the S-linked carbonyl, displacing the sulfur. Cathechols: Vibriobactin Vibriobactin is a siderophore used by Vibrio cholerae Its synthesis also involves a large, nonribosomal peptide synthetase, and follows many of the same pathways as the synthesis of hydroxamate siderophores outlined above. Vibriobactin HO HO O N Me O O NH Me N NH N OH O OH OH O OH The Cathechol Prosthetic Group The cathechol prosthetic group is 2,3dihydroxybenzoic acid, which is formed from chorismic acid Nonribosomal Peptide Synthetase 2,3-dihydrobenzoic acid then acts as a substrate for Vibirobactin Syntetase It is first activated by adenylation, then transferred to the enzyme with formation of a thioester Transfer to Norspermidine This thioester complex then undergoes nucleophilic attack by a primary amine on norspermidine. The norspermidine/cathechol complex goes on to react with two more cathechol prosthetic groups (these, however, attached by threonine derived linkages) to form the final siderophore Norspermidine+cathecol Me O NH N O OH OH H N NH 2 Yersiniabactin S S Me Me S Me N N H N OH OH COOH Synthesis Although it has neither hydroxamate nor cathechol groups, Yersiniabactin follows some of the same synthesis pathways, using a nonribosomal peptide sythetase that has clear homologies with, for example, vibriobactin sythetase Beginnings Synthesis begins with salicylic acid (2hydroxy-benzoic acid) This is activated by the attachment of an adenylate group, then loaded onto the enzyme by the formation of a thioester, as before Elongation At the same time, two cystines are also activated then loaded onto the same enzyme, also via a thioester linkage Then, in the condensation/cyclization domain, the salicyate group is transferred onto one of the cystines, which is then cyclized. This cyclization is an unusual property of this particular synthetaes Completion A second cystine is added, and also cyclized, and the resulting molecule undergoes the addition of a malonyl group, Sadenosylmethionine, and an additional cystine to complete the synthesis Overview: The use of a large, multidomain nonribosomal peptide synthetase was a common element of all of these syntheses. All of these processes included the activation of a substrate by adenylation and the transfer to a thioester linkage with the enzyme, followed by condensation to form a longer chain. This is similar to the process followed in biosynthesis of fatty acids. References Roosenberg, J.M. and Miller, M.J. Total Synthesis of the Siderophore Danoxamine. J. Org. Chem. 2000 Vol. 65 No. 16. 4833 – 4838. Lin, Y. and Miller, M.J. Synthesis of Siderophore Components by and Indirect Oxidation Method. J. Org. Chem. 1999 Vol. 64 No. 20. 7451 – 7458. Gaspar, M., Grazina, R., Bodor, A., Farkas, E., and Santos, M.A. Macrocyclic tetraamine tris(hydroxamate) ligand. J. Chem Soc. 1999 799 – 806. Duhme, A.K. Synthesis of two dioxomolybdenum complexes of a siderophore analogue. J. Chem. Soc. 1997 773 – 778. Atkinson, A. Bacterial Iron Transport. Biochemistry. 1998. 15965 15973