* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download drug-design
Cre-Lox recombination wikipedia , lookup
List of types of proteins wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Biochemistry wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
De novo protein synthesis theory of memory formation wikipedia , lookup
Bottromycin wikipedia , lookup
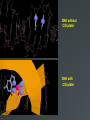
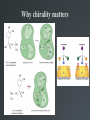
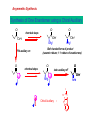
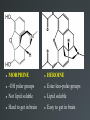
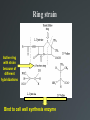
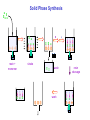
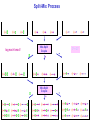
Drug Design Geometrical isomerism Chirality Chiral auxiliaries synthesis Polarity & Modifications Ring strain Combinatorial chemistry Parallel synthesis Geometrical Isomerism Against cancer Inactive DNA without CIS-platin DNA with CIS-platin Chirality Thalidomide Why chirality matters Asymmetric Synthesis Synthesis of One Enantiomer using a Chiral Auxiliary O O O chemical steps OH NH 2 OH OH NH 2 Bothhandedforms of product (racemic mixture; 1:1mixtureof enantiomers) Put auxiliary on O O chemical steps O takeauxiliary off OH NH 2 NH 2 O HN [ Chiral Auxiliary : O ] Polarity MORPHINE HEROINE -OH polar groups Ester less-polar groups Not lipid soluble Lipid soluble Hard to get in brain Easy to get in brain Modifying polarity Amine + HCl => hydrochloride salt (ionic, more soluble) Prozac Modifying polarity Carb. Acid + NaOH => Sodium salt (ionic, more soluble) Aspirin Esters as prodrugs Prodrug Drug Fatty barrier esterase O C R O C C R O O Prodrug O OH O Drug O O C C R O Ester masking polar groups allowing passage through fatty cell membranes esterase R OH Ring strain Active ring with strain because of different hybridizations Bind to cell wall synthesis enzyme What is Combinatorial Chemistry? • Is an approach that provides efficient synthesis of a large collection of molecules • Screening of libraries of related compounds to isolate the molecule of desirable property • Used in both academia and industries to generate huge libraries of compounds that have important biological properties Combinatorial Chemistry Combinatorial Library • • • • 4 monomers Nn Combinations 44 = 256 tetramers Solid Phase Synthesis resin + monomer shake wash wash resin cleavage Split-Mix Process tag each bead! Mix-Split Couple Mix-Split Couple 33= 27 Solid Phase Library In 1991s, Houghten & Lam: synthesis of a huge peptide library 20 amino acids Solid-phase synthesis 202 = 400 dipeptides DNA: fully automatic (solution) peptide 203 = 8000 tripeptides 204 = 160,000 tetrapeptides carbohydrate small molecule (drug-like) ln 1992, Jon Ellman: synthesis of non-peptide drug-like molecules by solid phase synthesis Parallel synthesis On a teflon reaction block Large number of wells Add different parts at each step Common conditions 12-well reaction block Add Scaffold to each well S S S S S S S S S S S S Wells after Addition of first reagent SA SB SC SD SA SB SC SD SA SB SC SD There are now twelve different products SA1 SB1 SC1 SD1 SA2 SB2 SC2 SD2 SA3 SB3 SC3 SD3 Computer aided drug design