* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Maneeshi Prasad
Behavioral epigenetics wikipedia , lookup
Protein moonlighting wikipedia , lookup
Cancer epigenetics wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Non-coding RNA wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Neurobiological effects of physical exercise wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Gene expression programming wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Gene expression profiling wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Messenger RNA wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Epitranscriptome wikipedia , lookup
Primary transcript wikipedia , lookup
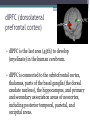
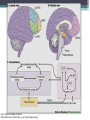
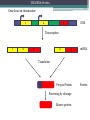
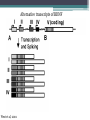
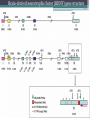
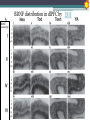
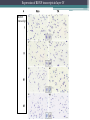
BDNF Seminar Spring 2010 Maneeshi Prasad Jan 29th 2010 dlPFC (dorsolateral prefrontal cortex) • dlPFC is the last area (45th) to develop (myelinate) in the human cerebrum. • dlPFC is connected to the orbitofrontal cortex, thalamus, parts of the basal ganglia (the dorsal caudate nucleus), the hippocampus, and primary and secondary association areas of neocortex, including posterior temporal, parietal, and occipital areas. Jon S. Simons & Hugo J. Spiers Nature Reviews Neuroscience 4, 637-648 (August 2003) dlPFC • dlPFC serves as the highest cortical area responsible for motor planning, organization, and regulation. • It plays an important role in the integration of sensory and mnemonic information and the regulation of intellectual function and action. • It is also involved in working memory. • Complex mental activities require additional cortical and subcortical circuits that are connected with dlPFC. • dlPFC development and maturation may last up to two decades in humans and may thus show differential expression of genes. BDNF • Brain-derived neurotrophic factor belongs to "neurotrophin" family of growth factors • BDNF support growth and differentiation of new neurons and synapses in CNS and PNS • BDNF is active in the hippocampus, cortex, cerebellum and basal forebrain—areas vital to learning, memory, and higher thinking • BDNF is also involved in neurogenesis • BDNF binds to TrkB receptor and p75NTR receptor BDNF in CNS • Alternative promoter usage provides differential mRNA stability and subcellular localization • Promoter IV of BDNF has been implicated in forming inhibitory synapses in the cortex • BDNF mRNA with long 3’UTR are localized in dendrites of cortical neurons, while short 3’UTR mRNA is restricted to soma • Loss of long 3’UTR mRNA results in denser and thinner dendritic spines of CA1 pyramidal neurons and reduced hippocampal longterm potentiation • Rat visual cortex shows expression of transcripts III-V and IV-V in cell soma, while IV-V is expressed in dendritic processes BDNF in CNS • Early postnatal development shows greater increase in spine density which is reduced by ~40% in later life • Conversion of proBDNF to mBDNF promotes late-phase long-term potentiation expression in hippocampus • ProBDNF is higher during postnatal stages while mBDNF is prominent in adults Yang, J. et al. (2009) Nat. Neurosci. 12:113. BDNF during development • Plays a important role during cortical development and is required for formation of ocular dominance columns in visual cortex • High levels of BDNF mRNA its TrkB receptor has been seen in dlPFC of young adults which subsequently decreases in adults DNA-RNA-Protein Gene locus on chromosome 1 2 3 DNA Transcription 1 2 3 3 mRNA Pre-pro Protein Protein 2 Translation Processing by cleavage Mature protein Alternative transcripts of BDNF West et al, 2001 Brain-derived neurotrophic factor (BDNF) gene structure Human BDNF gene structure • 10 noncoding exons in the 5’UTR • Presence of multiple translational (ATG) start sites The Brian Table. 1. Brain cohort demographics Demographic variables • Postmortem pH values: 6.12-6.98 • Postmortem Interval (PMI): 4 hr-32 hr • RNA integrity (RIN) varied Expression of housekeeping genes across development No variation in mRNA expression of housekeeping genes with changes in pH and RIN BDNF mRNA expression in dlPFC during development BDNF mRNA expression in dlPFC during development • Transcript I-IX changed significantly across development ▫ ▫ ▫ ▫ Higher during earlier stages Peaked at infancy Decreased after infancy Constant level was maintained from school age till adulthood BDNF mRNA expression in dlPFC during development • Transcript II-IX was low at birth, increased during 1st few years and peaked in toddlers ▫ Lowest in neonates ▫ Increased in infants and toddlers ▫ Decreased during school age, similar to infants, and was maintained till adulthood BDNF mRNA expression in dlPFC during development • Transcript IV-IX highest in infants and toddlers ▫ Lowest in neonates ▫ Increased in infants and toddlers age group ▫ Decreased gradually from school age and stayed consistent from adolescent till adulthood BDNF mRNA expression in dlPFC during development • Transcript VI-IX peaks within first years of life ▫ Highest at infancy ▫ Decreased subsequently from toddler age till adulthood Real-Time PCR v BDNF Protein levels BDNF protein expression in dlPFC during development • Both proBDNF (28 kDa) and mature BDNF (14 kDa) bands were seen at all ages • Protein expression increased from neonates to infants • Infants had highest level of BDNF expression followed by toddlers • Mature BDNF form varied across development and peaked at infancy • Increase of protein level in toddler age group might be related to increase in level of IV-IX or II-IX transcripts • Decrease in protein levels in adults matches with decrease in mRNA levels BDNF distribution in dlPFC by ISH BDNF transcript Expression of BDNF transcripts in layer IV BDNF transcript Expression of BDNF transcripts in layer V & VI BDNF transcript BDNF distribution in dlPFC • All 4 BDNF transcripts were highest in deeper cortical layers V and VI ▫ ▫ ▫ ▫ Layer I: no expression at any age group Layer II: moderate expression Layer III: robust expression was seen in neonates Layer IV/mid cortical layer: lower expression neonates and low to moderate expression in older age group ▫ Layer V and VI: intense staining for BDNF seen in neurons Discussion • Transcripts I-IX, IV-IX and VI-IX had highest expression patters in infancy • II-IX transcript was highest at toddler age group and was delayed by 2-3 years as compared to the other 3 transcripts • Lower expression of all transcripts during school age years Discussion • DLPFC layer IV showed increased BDNF signal • DLPFC layer IV is enriched in inhibitory neurons: cannot express BDNF by itself • This BDNF signal may be due to the BDNF mRNA that is targeted to the apical dendrites of layer V pyramidal neurons Discussion • High level of BDNF transcripts & protein during early years and overlaps with 1.5 fold increase in synaptic density • Synaptic density may decrease during adolescence and stabilize by young adulthood with cortex reaching maturity, which overlaps with the decrease in BDNF levels Discussion • Current study differs from a previous study (Webster, 2002), where BDNF expression was lowest in infancy and higher in young adult group • This may be due to combining of neonatal and infant groups, and differences in cohorts Conclusions • The dynamic regulation of BDNF gene in hDLPFC may be activated in a promoter-specific manner • During postnatal cortical development, neuronal morphology and synaptic density may be regulated by transcript specific BDNF expression