* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Amanda Henke - USD Biology
Survey
Document related concepts
Transcript
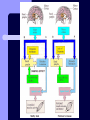
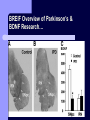
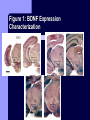
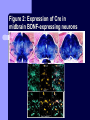
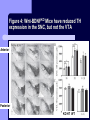
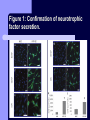
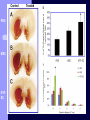
BDNF and Parkinson’s Disease March 26th, 2010 What is Parkinson’s Disease? Progressive loss of dopaminergic neurons in the substantia nigra Reduction in SN and striatal DA Increase in glial cells in the SN Neuromelanin (DA pigment) loss Lewy bodies Diagnosis Clinical features: Bradykinesia, resting tremors, muscle rigidity, loss of postural reflexes, flexed posture, and the freezing phenomenon – Parkinsonism diagnosis with 2 symptoms Parkinsonisms: – Primary: Parkinson’s disease (PD) – most common asymmetrical onset of motor symptoms rest tremor Substantial clinical response to levodopa therapy – Secondary: drug-induced or postencephalitic parkinsonism – Parkinson-plus syndromes - w/ other neurological features, i.e. progressive supranuclear palsy and multiple system atrophy – heredodegenerative disorders – parkinsonism features in a heritable degenerative disorder (juvenile Huntington or Wilson disease) Fahn and Sulzer, 2004 Neurotrophin Hypothesis in Neurodegenerative (ND) disorders Neurotrophins promote: development, heath, survival of neurons – BDNF: synaptic plasticity, neuronal survival and differentiation Studies suggest BDNF disruption in: – – – – Huntington’s Alzheimer’s Multiple Sclerosis Parkinson’s BREIF Overview of Parkinson’s & BDNF Research… Postmortem studies of PD patients: reduced levels of BDNF in the SCN- substantia nigra pars compacta (Mogi et al., 1999; BDNF Parain et al., 1999; Howells et al., 2000; Chauhan et al. 2001) BDNF promotes survival & differentiation mesencephalic DA neurons in culture (Hyman et al., 1999; Feng et al., 1999) BDNF protects from toxic insults (Murer et al., 2001) BDNF+/- mice have decreased striatal DA and impaired behavioral responses (Dluzen et al., 2001, 2002) trkB partial deletion – decreased TH, formation of α-synuclein deposits (von Bohlen Und Halbach et al., 2005) Normal BDNF Expression DA neurons normally co-express BDNF in: – – – Substantia Nigra Ventral Tegmental Area Frontal cortex DA neuron depletion Decrease in BDNF (trophic support)? Exogenous BDNF Replacement Goal: increase BDNF to preserve DA neurons and improve disease symptoms Problems: – – – – – Large molecular size (~28 kDa) trkB wide distribution – no targeted effects Carrier molecules: stem cells, viral vectors, biomaterials Unknown treatment length for protection, BDNF delivery rate, BDNF pharmokinetics BDNF overexpression in animal models seizures Experimental therapeutic strategies for restoring BDNF in ND diseases Zuccato and Cattaneo, 2009 Brain-Derived Neurotrophic Factor Is Required for the Establishment of Proper Number of Dopaminergic Neurons in the Substantia Nigra Pars Compacta Baquet et al., 2005. Journal of Neuroscience. 25(26): 6251-6259. Aim of Study Investigate the link between reduced BDNF in the substantia nigra and deterioration of dopamergic neurons in PD patients. Create a conditional knock-out, as BDNF-/mice die. Cre-Lox recombination Wnt-1 promoter R26R Cre (BDNFneo) LacZ Resulting Mice BDNF-/- BDNF+/- Wnt-1:R26R Wnt-BDNFKO BDNFneo/lox+ Heterozygous for BDNF BDNF+/+ Wildtype BDNF Figure 1: BDNF Expression Characterization What is TH? Kreek, et al. 2002 Figure 2: Expression of Cre in midbrain BDNF-expressing neurons Figure 3: Reduced BDNF protein leads to motor deficits and reduced striatal TH in Wnt-BDNFKO KO HT WT KO HT WT Figure 4: Wnt-BDNFKO Mice have reduced TH expression in the SNC, but not the VTA Anterior Posterior KO HT WT Figure 5: No change in NeuN, CB, CR NeuN CB CR Conclusions Selective BDNF deletion from the midbrain & hindbrain show: – – – reduced TH (differentiated DA neurons) reduction in striatal DA display early PD phenotype More evidence for a link between BDNF and PD? Protective Effects of Neurotrophic Factor-Secreting Cells in a 6-OHDA Rat Model of Parkinson Disease Sadan et al., 2009. Stem Cells and Development. 18(8):1179-90. Aim of study Induce MSC to differentiate into neurotrophic factor secreting astrocytes – – Safe & efficient protocol Increase NTF secretion Study effects NTF (BDNF and GDNF) in: – – – behavior dopamine levels/neurons in striatum in vivo tracking of transplanted cells Definition of a Stem Cell 1. make identical copies of themselves for long periods of time (longterm self-renewal) 2. give rise to mature cell types that have characteristic morphologies (shapes) and specialized functions 8-cell stage Why use stem cells for ND therapy? 1. 2. Replacement of degenerated cells Improve the environment of diseased neural tissue – i.e. release neuroprotective factors 3. Factors already secreted by stem cells Specific gene introduction to stem cells for secretion Stem cells to induce/enhance neurogenesis to mimic native stem cell populations Obstacles in stem cell therapy Immune (graft) rejection Transplantation procedure Risk of tumor development Ethical issues Matched donor Fate assessment after therapy Types of Stem Cells Embryonic Stem Cells (ESC) – totipotent – – – Adult stem cells – many types, multipotent – Ethical issues Tumorigenic Non-autologous source Different properties induced Pluripotent stem cells – – – – Autologous source Unlimited differentiation Tumorigenic Lentivirus vectors for induction – dangerous mutations Safer method = piggyBac Bone marrow stem cells Hematopoietic stem cell N e u r o n G l i a Neural Opposition to idea of MSC transdifferentiation to neuronal cells Observations of extending neurites mistaken for cell-cell contacts ‘neural’ makers could have different roles in MSC Yet, recent reports suggest a subpopulation of MSC originate from the neural crest – likely that at least of subset of the MSCs may have a neural predisposition. MSC advantages Differentiate to DA neurons, astrocytes, oligodendrocytes Paracrine effect – Secrete soluble trophic factors (BDNF, VEGF, GDNF) Cytokine secretion to inhibit lymphocyte proliferation Migratory behavior Neurogenesis – seen in stroke model and transplant to dentate gyrus of hippocampus, attributed to NTF secretion Genetic manipulations to overexpress genes or program cells MSC induction to NF-SC Human Mesenchymal Stem Cells Passaged 1218 days SPN L-glutamate dbcAMP IBMX N2 hEGR hbFGF PDGF HRG1-β1 hbFGF Media replaced 72 hrs later Neurotrophic factor secreting cells Figure 1: Confirmation of neurotrophic factor secretion. In vitro model of Parkinson’s Serum-free media (control) MSC Culture supernatant (control) Serum-free media NTF-SC Culture supernatant (contains NTF) Serum-free media + 1h 32-160 μM 6-OHDA 6-OHDA 6-hydroxydopamine – selectively neurotoxic for DA neurons drug redistributes DA from synaptic vesicles Oxidized DA = DA-quinone reacts w/ DA uptake transporter Figure 2: NTF-SC/MSC protect neuroblastoma cells against 6-OHDA toxicity • MSC and NTF-SC groups were statistically significant @ 32, 48, and 72μM 6OHDA. • No statistical difference b/t MSC & NTF-SC • @ 160μM, NTF-SC were statistically different from others Figure 3: Behavioral tests after stem cell transplant in 6-OHDA treated rats Control PBS MSC NTFSC Treated % Dopamine in lesioned compared to control side Cellular transplantation inhibited 6OHDA-induced dopamine depletion 100 * 90 80 70 60 50 40 30 20 10 0 Control MSC NTF-SC Conclusions and Future Directions NTF-SC could – – – increase production/ secretion of BDNF & GDNF Attenuate 6-OHDA-induced behavior Increase striatal dopamine Autotransplantation of rat-derived MSC and induced NTF-SC Transplantation later and at a site further from the lesion Treatment for PD Baquet et al., 2005 S Fig 1 Baquet et al., 2005 S Fig 2 Baquet et al., 2005 S Fig 2 Sudan et al, 2009 S Fig 1 S Fig 2 S Fig 3 Gene’s associated with early onset PD α-synuclein UCHL1 (ubiquitin carboxy-terminal hydrolase L1) Parkin – ubiquitin E3 ligase that prepares proteins for degradation DJ1: a parkin associated protein involved with oxidative stress PINK1: Phosphatase and tensin homolog–INduced Kinase; putative serine threonine kinase possible pathogenic mechanisms? PD Etiology 10% of cases: genes – – – α-synuclein Parkin DJ-1 90% of cases – unknown – – Age Environment (toxic exposure, drug use)