* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Isotonic Hypotonic Hypertonic
Cell nucleus wikipedia , lookup
Tissue engineering wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell growth wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell culture wikipedia , lookup
Signal transduction wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell membrane wikipedia , lookup
Cytokinesis wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
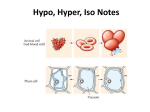
Chemistry of Life (157 – 163) 6.3 Cell Processes Active and Passive Transport, Osmosis, Diffusion, Exocytosis, and Endocytosis pp. 155 – 156; 195 -200 6.2, 8.1 Hickox: Baker High School Biology Most membranes cell functionsare involve chemical reactions. Food molecules into cells to provide the chemical constituents needed synthesize molecules. four main biochemicals: Cell selectively permeable. This gives themtaken the ability toreact maintain cell homeostasis by regulating whattoenters and other leaves the cell. There The are direction of water movemeC Objective 2.0: Describe cell processes necessary for achieving homeostasis, including active and passive transport, osmosis, diffusion, exocytosis, and endocytosis • Identifying functions of carbohydrates, lipids, proteins, and nucleic acids in cellular activities • Comparing the reaction of plant and animal cells in isotonic, hypotonic, and hypertonic solutions • Explaining how surface area, cell size, temperature, light, and pH affect cellular activities • Applying the concept of fluid pressure to biological systems Examples: blood pressure, turgor pressure, bends, strokes Core concept: Vocabulary Most cell functions involve chemical reactions. Food molecules taken into cells react to provide the chemical constituents needed to synthesize other molecules. There are four main biochemicals: Carbohydrates, Proteins, Lipids and Nucleic acids. Cells use carbohydrates to provide energy. Cells use proteins for cellular construction and repair, cellular chemical activities, and as a back-up energy source if carbohydrates are not available. Both breakdown and synthesis are made possible by a large set of protein catalysts, called enzymes. Cells use lipids for cell membrane construction. Cells use nucleic acids to store genetic information for protein synthesis. • • • • • Core concep: Cell membranes are selectively permeable. This gives them the ability to maintain cell homeostasis by regulating what enters and leaves the cell. The direction of water movement across the cell membrane depends on the relative concentrations of free water molecules in the cytoplasm and in the fluid outside the cell. Different kinds of cells have evolved different ways of dealing with hypertonic, hypotonic, and isotonic solutions. The cells of plants have rigid cell walls that keep the cells from expanding too much. Animal cells can avoid swelling by moving dissolved particles from the cytoplasm. Carbohydrate Nucleic Acid Lipids Proteins Enzyme Vocabulary • Passive transport •Active transport •Endocytosis •Isotonic •Exocytosis •Diffusion •Hypotonic •Hypertonic •Homeostasis •Osmosis Core Concept: Small cells function more efficiently than large cells. Small cells can exchange substances more readily than large cells because small objects have a higher surface-area-to-volume ratio than larger objects. Most cells function best within a narrow range of light, temperature and pH. At very low temperatures, reaction rates are too slow. Extremes of light, temperature and pH can irreversibly change the structure of most protein molecules. Vocabulary: pH, surface area, volume Turgor pressure is the main pressure of the cell contents against the cell wall in plant cells and bacteria cells, determined by the water content. Blood pressure refers to the force exerted by circulating blood on the walls of blood vessels. High blood pressure can cause stroke. Breathing gas under pressure can present a myriad of possible medical problems. One of these is decompression sickness or "the bends," caused by breathing nitrogen or other gases under pressure, which are not metabolized by the body. Vocabulary • Bends • Stroke Hickox: Baker High School Biology Simple to Complex Life’s Levels of Organization Our journey begins here, atoms like Hydrogen (H) and Oxygen (O) Atoms come together to male up molecules like water, H2O. Hickox: Baker High School Biology Chemical Reactions REACTANTS Sun CO2 + H2O CHEMICAL EQUILIBRIUM PRODUCT(S) C6 H12 O6 + 02 Acids and Bases • Acids are substances that forms in water and release hydrogen ions (H+). • Bases are substances that either take up hydrogen ions (H+) or release hydroxide ions (OH-). • pH measures how acidic or basic a solution is Hickox: Baker High School Biology pH Scale • A pH scale is used to indicate how acid and basic of a solution. – Ranges from 0-14 • 7 = Neutral • >7 = Base • <7 = Acid Hickox: Baker High School Biology Hickox: Baker High School Biology pH Matters A strong acid is pH of 2 pH is a measure of proton (hydrogen ion or H+) concentration . Low pH = lots of H+s, high pH = few H+s. In biology, keeping H+ levels within a narrow range is critically important. A strong base is pH of 12 Essential Question What are differences and similarities of Osmosis and Diffusion? Hickox: Baker High School Biology VIDEO FROM FILE Diffusion Diffusion is the net movement of particles from an area of higher concentration to an area of lower concentration. Diffusion continues until there is no concentration gradient! What affects the speed of Diffusion? • concentration: main factor, higher the more • temperature: if temp. increase greater diffusion • pressure: increasing pressure increases diffusion Dynamic equilibrium no concentration gradient! Dynamic equilibrium Osmosis Hickox: Baker High School 13 Your turn: Compare Osmosis and Diffusion Osmosis Diffusion Hickox: Baker High School Biology Osmosis • Isotonic Solution - Solute and Solvent (water) concentrations both inside and outside the membrane are equal. • Hypotonic Solution - Solution with a lower concentration of solute than the solution on the other side of the membrane. – Cells placed in a hypotonic solution will swell. • Lysis Hickox: Baker High School 15 Osmosis • Hypertonic Solution - Solution with a higher concentration of solute than the solution on the other side of the membrane. – Cells placed in a hypertonic solution will shrink. • Plasmolysis Hickox: Baker High School 16 The results of diffusion When a cell is in dynamic equilibrium with its environment, materials move into and out of the cell at equal rates. As a result, there is no net change in concentration inside or outside the cell. Material moving out of cell equals material moving into cell What type of osmosis is this? (Isotonic, hypotonic, or hypertonic) Does cell shrink, expand, or stay the same? What type of osmosis is this? (Isotonic, hypotonic, or hypertonic) Does cell shrink, expand, or stay the same? What type of osmosis is this? (Isotonic, hypotonic, or hypertonic) Does cell shrink, expand, or stay the same? VIDEO FROM FILE Your Turn: OSMOSIS ______________ is the diffusion of particles through a semipermeable membrane. __________________: movement of particles across cell membranes by diffusion or osmosis. The cell uses NO energy to move these particles __________________: the transport of materials against the gradient and this takes energy ___________________: the pushing out of water in a plant cell against the cell wall ___________________: shrinking of cell due to water moving out (dissolved solution outside cell high) _____________: swelling of a cell due to water moving in ____________: concentrations inside and outside cell is balanced. Diffusion in Living Systems: the way cells move substances in and out of the cell Osmosis is the diffusion of particles through a semi- permeable membrane. Hypotonic Isotonic Hypertonic Essential Question What are the essential molecules of Life? Hickox: Baker High School Biology Biomolecule: Structures vary RINGS Three views of glucose, a common simple sugar. BUNCHED CHAINS amino acids lipids LONG CHAINS Molecules of Life How do you build a cell? Start with water, add lots of small carbon-containing molecules and ……. Use these four major classes of biological molecules: Carbohydrate, lipid, protein, nucleic acid. Carbohydrates Carbon, Hydrogen, and Oxygen Carbohydrates are used for energy and to create structures. The building blocks for carbohydrates are simple sugars. Three views of glucose, a common simple sugar. Lipids are large and are made mostly of carbon and hydrogen and small amounts of oxygen. Fat Lipids do not dissolve in water. Lipids are used by cells for energy storage, insulation, and protective coatings, such as in membranes. Phospholipid Phospholipids Form Double-Layered Biological Membranes Protein p. 66 Proteins are THE key elements of life. Proteins provide structure for tissue and organs and carry out cell metabolism. Provide the body with the ability to move muscles. They are needed to transport oxygen in the bloodstream. Proteins are large and complex; made of carbon, hydrogen, oxygen, nitrogen and sometimes sulfur. Enzymes, a type of protein that change the speed of chemicals reactions in the body. An enzyme (p. 66) is a protein that enables other molecules to undergo chemical changes to form new products. Enzymes increase the speed of reactions that would otherwise proceed too slowly. Substrate Active site Strands of the Protein Keratin Create Hair Nucleotides are Chains of Linked Amino Acids DNA and RNA The building blocks of proteins are amino acids! There are two kinds of nucleic acids, DNA and RNA. Both are involved in the storage and flow of information from gene to gene product. Nucleotides Are the Monomers That Create Polymers of DNA and RNA The small subunits that make up nucleic acids are nucleotides. DNA Hickox: Baker High School Biology RNA Mader: Biology 8th Ed.