* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PPT File
Discodermolide wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Stille reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Kinetic resolution wikipedia , lookup
Elias James Corey wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
George S. Hammond wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Petasis reaction wikipedia , lookup
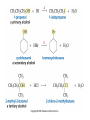
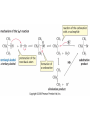
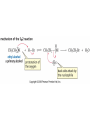
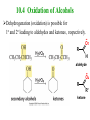
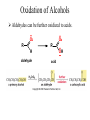
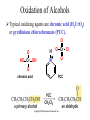
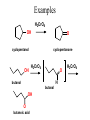
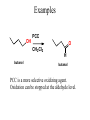
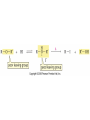
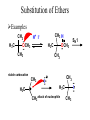
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 10 Reactions of Alcohols, Amines, Ethers, and Epoxides 10.1 Nomenclature of Alcohols Compounds in which a hydrogen is replaced by an OH group. We distinguish three types: Naming Alcohols Common names are the name of alkyl group followed by the word “alcohol” Naming 1. Longest carbon chain containing the alcohol. 2. OH suffix gets the lowest possible number. Naming 3. Find also lowest possible number for other substituent. 4. For more than one substituent they are listed in alphabetical order. 10.2 Substitution Reactions of Alcohols Alcohols are not reactive in nucleophilic substitution or elimination reactions since hydroxide is a strong base, poor leaving group. R OH Nu R OH X X R+ Nu no reaction in either case + R HO- + strong base SN1 HO- SN2 poor leaving group Substitution Reactions of Alcohols The situation improves under acid conditions. We change the leaving group to water, a neutral group. R OH H+ R O H NuNu R + H neutral O H neutral H - H2O R+ NuNu R 10.3 Elimination Reactions of Alcohols Elimination of water, dehydration, is commonly obtained using sulfuric acid (H2SO4) as a catalyst. H3C OH CH3 H2SO4 H3CHC CHCH3 + H2O The acid is mandatory to convert the poor leaving group OH– into a good leaving group H2O. Elimination First step is protonation of the hydroxyl group. H H3C OH H2SO4 CH3 H3C O H CH3 Loss of water leads to a carbocation. H H3C O H - H2O CH3 H3C CH3 Elimination Second, a base removes a proton b to the carbocation center. H3C b H H H3CHC CHCH3 CH3 + H2SO4 OSO3H Notice that this reaction is an E1 reaction. •Rate-determining step is the formation of the carbocation. Elimination In case we have a choice between several bhydrogens, the most stable alkene is formed preferentially. CH3 H3PO4 H3C CH3CHCH2CH3 OH H3C CH3 H2C CH + H3C 84 % CH2 CH3 16 % Elimination As a result of the E1 mechanism, the ease of dehydration follows the order: R H R R OH > H OH > R H R R OH That directly reflects the stability of the intermediate carbocations. R R R H R > H R > R H Elimination Primary alcohols undergo dehydration by an E2 pathway. First, however, we generate the good leaving group. H + OH H O H The subsequent steps, removal of water and deprotonation, take place simultaneously. OSO3H H H3C CH H O H H3C CH2 10.4 Oxidation of Alcohols Dehydrogenation (oxidation) is possible for 1o and 2o leading to aldehydes and ketones, respectively. O R H aldehyde O R R' ketone Oxidation of Alcohols Aldehydes can be further oxidized to acids. O R O R H aldehyde OH acid Oxidation of Alcohols Typical oxidizing agents are chromic acid (H2CrO4) or pyridinium chlorochromate (PCC). O - O H O Cr Cl HO Cr OH N O O chromic acid PCC Examples H2CrO4 OH cyclopentanol OH O cyclopentanone H2CrO4 O H butanol butanal OH O butanoic acid H2CrO4 Examples OH PCC O CH2Cl2 butanol H butanal PCC is a more selective oxidizing agent. Oxidation can be stopped at the aldehyde level. 10.5 Reaction of Amines Amines are less reactive than alcohols. • This can be evaluated by inspection of the pKa values of the leaving group. pKa HF HOH HNH2 3.2 15.7 36 Protonation of the amine improves the situation only slightly. H H CH3CH2OH pKa -2.4 > CH3CH2NH2 11.2 Amines Amines are the most common organic bases. CH3 H NH CH2CH3 pKa 10.8 H NH H NH2 CH3 CH3 10.9 H NH 5.07 40 10.6 Nomenclature of Ethers Common Name: • Name of alkyl substituents followed by “ether” Nomenclature of Ethers IUPAC •Parent alkyl compound with RO substituent. “-yl” is then replaced by “oxy” 10.7 Substitution Reactions of Ethers The behavior of ethers is comparable to alcohols. • pKa of the leaving group is comparable. H OCH3 H OH pKa 15.7 15.5 Activation by acid allows substitution. H R O R' R O R' + HI R I + R' OH Substitution of Ethers The mechanism involves first a protonation step. H R O R' + HI R O R' The subsequent steps are determined by the stability of the intermediates. • Stable carbocation SN1 • Unstable carbocation SN2 Substitution of Ethers Examples CH3 H3C CH3 H H+ IH3C OCH3 CH3 OCH3 CH3 stable carbocation CH3 H3C SN1 CH3 I H3C CH3 attack of nucleophile I CH3 Substitution of Ethers H3C H+ IOCH3 H3C H OCH3 SN2 Primary carbocations are unstable; thus, reaction proceeds via SN2. H3C H O CH3 SN2 H3C OH + CH3I I Reaction takes place on the less hindered of the two alkyl groups. Ethers Only hydrogen halides react with ethers Ethers commonly used as solvents Often used solvents are: O O tetrahydrofuran THF O O tetrahydropyran 1,4-dioxan CH3CH2OCH2CH3 diethyl ether "ether" CH3OCH2CH2OCH3 dimethoxyethane DME