* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Ch. 16: Solutions - Quynh Nguyen Official Website
Elias James Corey wikipedia , lookup
Aldol reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Carbohydrate wikipedia , lookup
Hydroformylation wikipedia , lookup
Asymmetric induction wikipedia , lookup
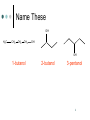
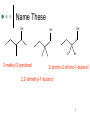
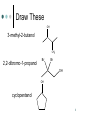
Ch. 12: Compounds with O and S Chem 20 El Camino College 1 Alcohols, Thiols, Ethers O H3C methanol O S H H3C H methanethiol H3C CH3 dimethyl ether 2 Naming Alcohols Name the longest carbon chain containing the OH group, use the –ol suffix Number the chain to give the OH the lowest number Give the location and name of other substituents in alphabetical order. 3 Name These OH H3C CH2 CH2 CH2 OH OH 1-butanol 2-butanol 3-pentanol 4 Name These OH OH OH Cl 3-methyl-2-pentanol Br 2-bromo-2-chloro-1-butanol 2,2-dimethyl-1-butanol 5 Draw These OH 3-methyl-2-butanol CH3 2,2-dibromo-1-propanol Br Br OH OH cyclopentanol 6 Naming Ethers Write the name of each group attached to the O Use alphabetical order If the groups are the same, use the prefix “di” O H3C CH3 CH2 H3C CH2 O CH2 O CH3 CH3 ethyl methyl ether diethyl ether cyclopropyl methyl ether 7 Solubility “Like dissolves like” explains solubility Alkanes are nonpolar they are insoluble in water Ethers are slightly polar ethers with small carbon chains are water soluble ethers with long carbon chains are water insoluble Alcohols are polar alcohols with small/medium C chains are soluble alcohols with large C chains are water insoluble. 8 Give Name: Solubility propane: insoluble in water O dimethyl ether: soluble in water O dibutyl ether: insoluble in water ethanol: soluble in water OH 1-hexanol: soluble in water OH 1-octanol: insoluble in water OH 9 Aldehyde and Ketone 10 Oxidation Reaction In one kind of oxidation rxn of alcohols, the carbon bearing the OH turns into a C=O group If the C bearing the OH is attached to one other carbon or less, an aldehyde forms If the C bearing the OH is attached to two other carbons, a ketone forms If the C bearing the OH is attached to three other carbons, no reaction happens. 11 O [O] O H3C H3C H O [O] O CH2 H3C H H aldehyde C H3C H CH H C H3C H O ketone [O] O aldehyde C CH3 H3C H3C C H3C [O] O H CH3 no reaction 12 Draw the product: aldehyde, ketone, or NR? O OH ketone [O] H aldehyde [O] HO O O OH [O] ketone 13 Naming Aldehydes Name the longest carbon chain containing the carbonyl (C=O) Use the suffix “al” Name any substituents by numbering the O carbonyl carbon as number 1 H O O C C H H3C O H H 2-methylpropanal methanal (formaldehyde) ethanal propanal 14 15 Name These Br H H O pentanal Br O 2,3-dibromobutanal 16 Naming Ketones Name the longest carbon chain containing the carbonyl (C=O) Use the suffix “one” Number the main chain from the end nearest the carbonyl. Name and number any substituent H 3C O O C C CH3 propanone (acetone) H3C O CH2CH3 butanone CH3 3-methylbutanone 17 Name These O H O O pentanal 2-pentanone 3-pentanone 18 Name These O O O CH3 Cl cyclopentanone Cl 3-heptanone 3,3-dichlorobutanone 19 Properties Aldehydes and ketones are slightly polar They tend to have similar bps to ethers of the same molar mass They tend to have much lower bps than alcohols, because alcohols are much more polar Aldehydes and ketones of 5 C atoms or less are soluble in water 20 Chemical Tests In Tollens’ test, an aldehyde is oxidized to a carboxylic acid. Silver also forms. CH3CHO + 2 Ag+ 2 Ag(s) + CH3COOH 21 22 Chemical Tests In Benedict’s test, an aldehyde that has an OH group on the next carbon forms a carboxylic acid. Red ppt also forms. RCHO + 2Cu2+ Cu2O(s) + RCOOH 23 24 Isomers Isomers are compounds with the same formula, but the atoms are attached differently “Stereoisomers” have the atoms arranged differently in space--they are related as mirror images. 25 Chiral Carbons Chiral carbons are tetrahedral carbons with four different attachments 26 27