* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download chapter 8 notes - 8.4 and 8.5 - APBio09-10
Biochemical cascade wikipedia , lookup
Magnesium in biology wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Lipid signaling wikipedia , lookup
Citric acid cycle wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Restriction enzyme wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Proteolysis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Catalytic triad wikipedia , lookup
Metalloprotein wikipedia , lookup
Biochemistry wikipedia , lookup
Biosynthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
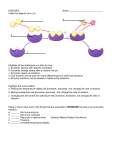
A. Sherman I. 8.4 – Enzymes speed up metabolic reactions by lowering energy barriers A. Enzyme – macromolecule that acts as a catalyst 1. Catalyst – chemical agent that speeds up reactions B. The activation energy barrier 1. All chemical reactions involve bond breaking and bond forming 2. For bonds to break, the starting molecules must be taken to a very unstable state 3. Activation Energy (free energy of activation) / E sub a / Ea a. is the energy required by the reactants to bring them to the unstable state in which their bonds can break b. Often absorbed from the surroundings as heat 4. When new bonds of product form, the energy is released back as heat 5. The so-called “unstable condition” is called the transition state 6. To get there: a. Ea is absorbed, usually as heat b. Molecular speed of reactants increases c. Bonds are more likely to break under heat 7. In exergonic reactions, Ea is repaid with interest since the formation of the news bonds releases more energy than what was needed to break the old bonds 8. Ea is usually very very high, and for a reaction to occur, reactants must be heated a lot (A lot of energy needs to be absorbed). C. How Enzymes Lower the Ea Barrier 1. Most complex molecules have lots of energy with potential to break down spontaneously 2. At cell temperatures, their activation energy is too high for random decomposition of molecules 3. Just heating reactants to cause reactions to occur is BAD because a. All reactions would speed up, not just the necessary ones b. Heat kills and denatures proteins 4. Catalysis – enzymes lower the Ea barrier 5. Enzymes cannot a. modify the overall change in energy of a reaction b. Make an endergonic reaction an exergonic one. 6. Enzymes DO a. Hasten reactions b. Make it possible for cells to have dynamic metabolisms c. Determine which process are going on in the cell D. Substrate Specificity of Enzymes 1. Substrate – reactant an enzyme acts on 2. Enzyme-substrate complex – formed when an enzyme binds to its substrate(s). 3. Enzymes can recognize their specific substrate even among isomers. a. Molecular recognition is possible because enzymes are proteins, each with a unique structure and sequence of amino acids that allows them to recognize their unique substrate(s). 4. Active site – region of enzyme that binds to substrate a. Formed by only a few of the enzyme’s amino acids 5. Induced Fit – when a substrate enters the active site, the enzyme changes shape for a snugger fit E. Catalysis in the Enzyme’s Active Site 1. Substrate is held in active zone with weak bonds 2. R groups of enzyme’s active zone catalyze reaction 3. Products leave, enzyme can take more substrates 4. Enzymes catalyze extremely fast and are never consumed 5. Enzymes can catalyze forward or reverse reactions, depending on which one has the negative energy change 6. How is the reaction sped up? a. For 2 or more reactants, the active site provides a template for substrates to come together in the proper orientation b. Enzyme stretches the substrate toward transition state form i. Stretches and bends chemical bonds that must be broken in reaction ii. Ea is directly related to the difficulty of breaking the substrate’s bonds iii. Distorting the bonds helps the substrate approach transition and lowers the free energy requirements c. Provides a more conducive microenvironment i. I.E: An enzyme’s R group may have acidic amino acids, and thus the active zone will be a pocket of low pH in an otherwise neutral cell ii. Active group can also be an H+ donor to aid catalysis d. Direct participation of the active site in the reaction i. Brief covalent bonds between substrate and enzyme may be formed ii. Enzyme is always returned to original form 7. Rate of Enzyme Conversion of Substrates a. More substrate molecules means more frequent access to enzyme active sites b. Saturation – occurs when all enzyme molecules have active sites engagted due to high substrate concentration i. Rate of reaction is directly proportional to the speed at which the active site converts substrates to products ii. The only way to increase the rate is to create more enzymes F. Effects of Local Conditions on Enzyme Activity 1. Optimal conditions favor the most active shape for enzyme molecule 2. Effects of temperature and pH a. Higher temp = higher rate ofr reaction i. Enzymes & substrates move faster and collide more ii. Above a certain temperature, the rate of reaction drops sharply i. Weak bonds that stabilize the active site become disrupted ii. Proteins denature iii. Ideal temp is around 35 – 40 C. b. Optimal pH i. Average: 6 – 8 ii. The optimal pH of Pepsin, an enzyme in the acidic human stomach, is 2 iii. Trypsin, in the intestine, catalyzes fastest at a pH of 8 3. Cofactors Non-protein “helpers” May be bound loosely May be permanent residents of enzyme Inorganic – Zinc, iron, copper ions Organic cofactors are called coenzymes i. Vitamins act as coenzymes or help form coenzymes 4. Enzyme Inhibitors a. If an inhibitors attaches itself with covalent bonds, inhibition is usually irreversible b. Most inhibitors bind via weak bonds and ARE reversible c. Competitive Inhibitors i. Resemble normal substrates and compete for the active site ii. Reduce enzyme productivity by blocking out real substrates iii. An be overcome by increasing substrate concentration i. More substrates get into enzymes than inhibitors d. Noncompetitive Inhibitors i. Impede enzymes by binding to non-active site ii. Active site becomes less effective if enzyme’s shape is changed (see 8.5) e. Toxins, Poisons, Antibiotics i. Sarin, DDT, and parathion are inhibitors of enzymes in the nervous system ii. Penicillin/other antibiotics block active sites of enzymes that bacteria use to make their cell walls. f. Enzyme Inhibition is not always bad or abnormal. It’s often used in regulation. 8.5 – Regulation of Enzyme Activity Helps Control Metabolism. A. Allosteric Regulation of Enzymes 1. Allosteric Regulation- A protein’s function at one site is affected by a regulatory binding at another. The regulatory molecules act like noncompetitive inhibitors. 2. Allosteric Activation and Inhibition a. Enzymes that are allosterically regulated i. Have 2 or more subunits ii. Each subunit has its own active site iii. The complex oscillates between inactive and catalytically active b. Activator – binds to enzyme, usually where subjoints join, and lock it into active form. c. Inhibitor – Binds to enzyme, locks it into inactive form d. Shape changes in 1 subunit cause the same shape change to all others i. A single activator or inhibitor changes the shape of the whole enzyme e. Fluctuating regulator concentrations control each other: i. ATP -> ADP and something else ii. ATP binds allosterically to enzymes that create ATP and inhibits their activity iii. ADP is in activator for the same enzymes iv. If ATP is plentiful, ATP inhibits its own making because that’s a waste of cell resources a. b. c. d. e. II. v. If ADP is plentiful, ATP will not be as plentiful, and ADP will activate enzymes that catalyze reactions that give off ATP. f. Cooperativity – One substrate primes all of an enzyme’s subunits for more substrates i. Hemoglobin is not an enzyme, but has 4 subunits i. The binding of one oxygen to hemoglobin increases hemoglobin’s affinity for oxygen ii. If oxygen is scarce, hemoglobin does not have this affinity because less oxygen molecules bind to hemoglobin ii. Aspartyl transcarbamoylase is a good example of cooperativity 3. Identification of Allosteric Regulators a. Regulatory sites between enzymes are more distinct than active sites b. Hard to identify allosteric regulators because they bind their enzyme at low affinity for its substrate and make the enzymes and regulators hard to isolate 4. Feedback Inhibition a. A metabolic pathway can be switched off like so: i. As an end product of a metabolic pathway accumulates, it will start inhibiting the first enzyme in its metabolic pathway because it is plentiful, and no more cell resources are needed to create more ii. Metabolic pathway is then switched off 5. Specific Localization of Enzymes within Cells a. Cells have compartments b. Cellular structures bring order to metabolic pathways c. Teams of enzymes can assemble and link i. One enzyme’s end product is another enzyme’s substrate d. Some enzymes have fixed locations e. Others are in solutions, enclosed with their own internal chemical environments.