* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Metabolic fate of amino acid
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Plant nutrition wikipedia , lookup
Microbial metabolism wikipedia , lookup
Catalytic triad wikipedia , lookup
Oligonucleotide synthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Point mutation wikipedia , lookup
Butyric acid wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Protein structure prediction wikipedia , lookup
Metalloprotein wikipedia , lookup
Peptide synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
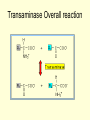
METABOIC FATE OF AMINO ACIDS • Intracellular proteases hydrolyze internal peptide bonds, of protein releasing peptides, which are then degraded to free amino acids by peptidases. • Endopeptidases cleave internal bonds, forming shorter peptides. • Amino peptidases and carboxy peptidases remove amino acids sequentially from the amino acids. • Extracellular, membrane-associated, and long-lived intracellular proteins are degraded in cellular organelles termed lysosomes by ATP- independent processes. • By contrast, degradation of abnormal and other occurs in the cystol. • Animals excrete nitrogen from amino acids and other sources as one of three end products: ammonia, uric acid, or urea. • Teleostean fish, which are excrete nitrogen as ammonia. ammonotelic, • Land animals convert nitrogen either to uric acid (uricotelic organisms) or to urea (ureotelic organisms)(Fig.11.1) Overall amino acids metabolisms • Urea biosynthesis is divided for discussion into 4 stages • (1) transamination, • (2) oxidative deamination of glutamate, • (3) ammonia transport, and • (4) reactions of the urea cycle. • These areas to the overall catabolism of amino acid nitrogen. • Free amino acids released from dietary or intracellular proteins are metabolized in identical ways. • Following removal of the a -amino nitrogen by transamination, the resulting carbon “Skeleton” is then degraded (1) Transamination • Transamination interconverts a pair of amino acids and a pair of keto acids, generally and a -amino acid and a a -keto acid (Fig.11.2). • While most amino acids undergo transmination, exceptions inculde lysine, threonine, and the cyclic imino acids proline and hydroxy proline. • Since transminations are freely reversible, transaminases (aminotransferases) can function both in amino acid catabolism and biosynthesis. • Pyridoxal phosphate resides at the catalytic site of all transaminases. • Alanine-pyruvate transaminase (alanine transaminase) and glutamate a -ketoglutarate transaminase (glutamate transaminase), present in most animals. Transaminase Overall reaction 1. Aspartate donates its amino group, becoming the aketo acid oxaloacetate 2. a-Ketoglutarate accepts the amino group, becoming the amino acid glutamate. (2) Oxidative Deamination • The a -amino groups of most amino acids ultimately are transferred to a -ketoglutarate by transamination, forming L-glutamate (Fig.11.3). • Release of this nitrogen as ammonia is then catalyzed by L- glutamate dehydrogenase that uses either NAD+ or NADP+ as oxidant. Oxidative deamination (3)Ammonia Transport by L-amino acid oxidases • L-amnio acid oxidase is present in liver and kideny tissue. • These autoxidizable flavoproteins oxidize amino acids to an a -imino acid that adds water and decomposes to the corresponding a -keto acid with release of ammonium ion (Fig.11.3). • The reduced flavin is reoxidized directly by molecular oxygen, forming hydrogen peroxide (H2O2), which is split to O2 and H2O by the enzyme catalase present in many tissues, especially liver. Ammonia transport by L-amino acid oxidase (4)Action of Glutamate Synthetase • While ammonia is constantly produced in the tissues, it is rapidly from the circulation by the liver and converted to glutamate, glutamine, and ultimately to urea. • Formation of glutamine is catalyzed by glutamine synthetase (Fig.11.5). • Synthesis of the amide bond of glutamine is accomplished at the expense of hydrolysis of one equivalent of ATP to ADP and Pi. Action of Glutamate synthetase (5)Action of Glutaminase and asparaginase • Hydrolytic release of the amide nitrogen of glutamine as ammonia, catalyzed by glutaminase, strongly favours glutamate formation. • Glutamine synthetase and glutaminase thus catalyze inter conversion of free ammonium ion and glutamine (Fig.11.6). • An analogous reaction is catalyzed by L-asparaginase. • These enzymes occur in liver, kidney and gills and also in red and white muscles. Action of Glutaminase and asparaginase (6)Urea Synthesis • Urea is the major end product of nitrogen catabolism in humans. • Urea is formed from ammonia, carbon dioxide, and aspartate, synthesis of I mol each of ammonium ion and the a -amino nitrogen of aspartate. • The biosythesis of urea involves 5 important steps 1. Synthesis of carbomyl phosphate Carbamoyl Phosphate Synthase (Type I) catalyzes a three-step reaction with carbonyl phosphate and carbamate intermediates. • Formation of carbamoy1 phosphate requires 2 mol of ATP • One ATP serves as a source of phosphate. • Conversion of the second ATP to AMP and pyrophophate, together with the coupled hydrolysis of pyrophophate to orthophosphate, provides the driving force for synthesis of the amide bond and the mixed acid anhydride bond of carbamoy1 phosphate. 2.Synthesis of citrulline • In the next step, carbamoy1 phosphate donates its carbamoy1 to ornithine to form citrulline and release phosphate in a reaction catalyzed by ornithine transcarbamoylase, a Mg2+ requiring enzyme. • The citrulline thus formed leaves the mitochondria and passes into the cytosol of the liver cells. 3.Synthesis of arginosuccinate • The transfer of the second amino group to citrulline occurs by a condensation reaction between the amino group of aspartate and the carbamoy1 carbon of citrulline in the presence of ATP to form arginosuccinate, catalyzed by the enzyme arginosuccinate synthetase. • The second amino group is introduced in the form of L aspartate, which in turn acquired it form L. glutamate by the action of aspartate transaminase. 4.Formation of arginine • In the next step, arginosuccinate is reversibly cleaved by arginosuccinate lyase to form free arginate and fumerate. • The fumerate so formed returns to the pool of citric acid cycle intermediates. 5. Formation of Ornithine • In the last reaction arginase cleaves arginase to yield urea and ornithine. • Ornithine thus generated enters the mitochondria again to initiate another round of urea cycle. Overall reaction • The overall equation of the urea cycle is • 2NH 4+ + HCO3- + 3ATP4- + H2O • Urea + 2AMP- + PPi3- + H+ • The urea cycle brings together two amino and HCO3- to form a molecule of urea which diffuses from the liver cells into the blood thence to be excreted into the urine by the kidneys. • Thus the toxic ammonia is converted into harmless urea in ureotelic animals. • Two molecules of ATPs are required to make carbamoy1 phosphate and • Two more molecules of ATP is required to make arginosuccinate. • In these reactions ATP undergoes a pyrophosphate cleavage to AMP and pyrophosphate, which may be hydrolysed to yield two orthophosphates. • Thus the ultimate cost for the formation of one molecule of urea is four ATPs.