* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download SARSIA
Epigenetics of neurodegenerative diseases wikipedia , lookup
Medical genetics wikipedia , lookup
Pathogenomics wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Essential gene wikipedia , lookup
Behavioural genetics wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Transposable element wikipedia , lookup
Genetic engineering wikipedia , lookup
Genomic imprinting wikipedia , lookup
Quantitative trait locus wikipedia , lookup
Ridge (biology) wikipedia , lookup
Koinophilia wikipedia , lookup
Frameshift mutation wikipedia , lookup
Gene expression programming wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Public health genomics wikipedia , lookup
Population genetics wikipedia , lookup
History of genetic engineering wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Gene expression profiling wikipedia , lookup
Designer baby wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Oncogenomics wikipedia , lookup
Minimal genome wikipedia , lookup
Genome editing wikipedia , lookup
Point mutation wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Genome evolution wikipedia , lookup
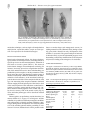
Short communication A mutational approach to the study of development of the protochordate Ciona intestinalis (Tunicata, Chordata) Paolo Sordino, Carl-Philipp Heisenberg, Paola Cirino, Alfonso Toscano, Paola Giuliano, Rita Marino, Maria Rosaria Pinto & Rosaria De Santis SARSIA Sordino P, Heisenberg C-P, Cirino P, Toscano A, Giuliano P, Marino R, Pinto MR, De Santis R. 2000. A mutational approach to the study of development of the protochordate Ciona intestinalis (Tunicata, Chordata). Sarsia 85:173-176. We have developed a protocol to perform a genetic screen for zygotic mutations affecting embryogenesis on the protochordate Ciona intestinalis. The choice of this taxon, whose phylogenetic position places it at the basis of the chordates as one the most primitive vertebrate relatives, could allow to address several evolutionary questions. The protochordates share many morphological features with the vertebrates, in primis the presence of a notochord. Ciona intestinalis shows several ideal features for a mutational analysis, such as external development and larvae made of a limited number of cells and cell types. Detailed cell lineage studies are available. The haploid genome size is comparable to the size of the Drosophila haploid genome. We have optimised conditions for chemical mutagenesis studying the efficiency at which different concentration of N-ethyl-N-nitrosourea (ENU) can induce mutations. Because the adult Ciona are hermaphrodites, we are performing a one-generation screen. The induced mutations are identified by visual inspection of developmental stages. We report the preliminary results from our screen including examples of the different classes of mutant phenotypes found so far. Paolo Sordino*, Carl-Philipp Heisenberg, Department of Anatomy and Developmental Biology, University College London, Gower Street, WC1E 6BT, London, United Kingdom. – Paola Giuliano, Rita Marino, Rosaria De Santis, Laboratory of Cell Biology, Stazione Zoologica Anton Dohrn, Villa Comunale, 80121, Napoli, Italy. – Maria Rosaria Pinto, Laboratory of Cell Biology, Stazione Zoologica Anton Dohrn, Villa Comunale, 80121, Napoli, Italy and Institute of Protein Biochemistry and Enzymology, CNR, Via Marconi 10, 80125, Napoli, Italy. * Corresponding author. E-mail: [email protected] Keywords: Ciona intestinalis; Urochordata; mutagenesis. INTRODUCTION In the last two decades, mutagenesis screens have strongly impacted upon our comprehension of developmental genetics, from early pattern formation to morphogenesis and behaviour. In a classic genetic approach, random mutagenesis makes it possible to survey the genome for genes that function in particular embryonic pathways. This approach allows the introduction of random mutations into individual genes causing phenotypic alterations which may be helpful to unravel the wildtype function of the mutated gene(s). Until now, mutagenesis screens applied to animal organisms have been focusing on two invertebrate and one vertebrate species. While for the nematode Caenorhabditis elegans and the insect Drosophila melanogaster these screens have led to the sequencing of a large number of molecules crucial in development (NüssleinVolhard & Wieschaus 1980; Chalfie 1993), the outcome of the mutagenesis screen in the zebrafish Brachydanio rerio (Mullins & al. 1994; Solnica-Krezel & al. 1994) has yet to await the identification of the majority of the mutated genes. The zebrafish has been chosen mainly on the basis that it is easy to keep and raise in large number under laboratory conditions and that zebrafish embryos are well suited for morphological examination and experimental manipulation during the whole course of embryonic development (Westerfield 1993). In the light of substantial recent progress in the establishment of genetic maps (Postlethwaite & al. 1998) and insertional mutagenesis methods (reviewed by Weinberg 1998), it is reasonable to expect that the cloning of mutated genes will be facilitated. The limitations of vertebrate screens may however become more evident by observations that many developmental control genes are found in multiple copies especially in the zebrafish genome, as a result of gene or subgenomic duplications or losses, indicating that there may be a high degree of partial redundancy for the function of these genes (see Wittbrodt & al. 1998). Therefore mutations in these genes 174 Sarsia 85:173-176 – 2000 ENU WT sperm egg self-fertilisation Fig. 1. Scheme for F1 genetic screen in C. intestinalis. N-ethylN-nitrosourea (ENU) was used to generate mutations (+*) in pre-meiotic spermatozoa. Adults belonging to the F1 progeny, obtained by crossing wild-type eggs with mutagenized spermatozoa, were self-fertilised and the F2 progeny was scored for morphological or heterochronic defects. may only lead to relatively mild phenotypes which could be the reason that a considerable portion of mutants may not have been identified in the zebrafish screens reported yet (Driever & al. 1996; Haffter & al. 1996). Developmental evolutionary biology is providing strong evidence that embryonic processes are conserved between basal chordates and vertebrates (e.g. Corbo & al. 1997; Shimeld 1997; Glardon & al. 1998; Marino & al. 1998). Consistent with this, an extension of the mutagenesis approach to the protochordate Tunicata, with their simpler genetics and development (Satoh 1994; Di Gregorio & Levine 1998), could facilitate dissection of programmes common for all chordates. While revealing parallels in developmental mechanisms, it can also offer the opportunity to uncover novel genes or gene functions and, ultimately, unravel the evolutionary emergence of vertebrate developmental processes. In an effort to generate zygotic mutations affecting embryogenesis in a protochordate species, we have established a methodology for inducing point-mutations in the ascidian Ciona intestinalis. Within the subphylum Tunicata, the ascidian class has been thoroughly studied in developmental biology most likely because of their sessility in coastal waters that makes sampling easier than for the pelagic classes Larvacea and Thaliacea (Jeffery & Swalla 1997). Ascidian larvae exhibit many vertebratelike anatomical characteristics, such as a dorsal neural tube, a notochord and tail muscles. Extensive lineage tracings, comparable to that in C. elegans, has been permitted by the simple, invariant embryonic cleavage patterns (Nishida 1987). Also, the small invertebrate-like genome of C. intestinalis (Lambert & Laird 1971; Simmen & al.1998) indicates that functional redundancy of genes may only be a minor problem for the identification of mutant phenotypes (Ohno 1970). One particular trait that separates C. intestinalis from other most popular research chordates is that it is hermaphrodite and self-sterile, but self-sterility can be abolished by removal of the egg coats or by controlled experimental conditions. This feature allows performing a one-generation scheme. Likewise, C. intestinalis, a rather cosmopolitan species, breeds throughout the year with a high rate of fecundity and its generation time is relatively short (2-3 months). These conditions can be reproduced in the laboratory following an uncomplicated culture method. In addition, the larva is very transparent and the cell lineage of the embryo has been described in detail (e.g. Nicol & Meinertzhagen 1988a, 1988b). Altogether, these represent ideal prerequisites for conducting a mutant screen. Moreover, several laboratories are expressing an interest in studying C. intestinalis, so that cloning of genes on the basis of homology to known developmental control genes in other vertebrate and invertebrate species, is progressing. MATERIAL AND METHODS To perform a pilot screen, adult Ciona were treated with ethylnitrosourea (ENU) (Russell & al. 1979; Yoshikawa & al. 1984; Zimmering & Thompson 1984; Grunwald & Streisinger 1992) and mutagenized chromosomes were driven to homozygosity employing a diploid F1 screen (Hitotsumachi & al. 1985; Riley & Grunwald 1995). First, pre-meiotically mutagenised sperm were crossed with wildtype eggs. Then, gametes from F1 individuals were self-fertilised and the offspring screened for morphological visible phenotypes at larval stages. Visual inspection of larval morphologies permitted the identification of several recessive lethal mutants (Fig. 1). Specific locus mutations were consistently represented in mendelian ratios and were transmitted through the germ lines of F1 offspring, suggesting that they have been generated by point-mutations. As for other favourite model systems, the experimental analysis of mutant tunicate larvae can be approached by several cellular and Sordino & al. – Genetic screen for zygotic mutations 175 Fig. 2. Examples of embryonic phenotypes generated during the pilot screen. Tubulin labelled 1 day old larvae. Compared to wild-type larva (A), the mutant big ocellus (bio) (B) is characterised by an expanded neural vesicle (black arrows) with an enlarged ocellus (arrowhead), while blind (bli) (C) shows no sign of pigmentation in the sensory cells (white arrow). molecular techniques, such as single-cell manipulations and injection, expression studies, ectopic or tissue-specific over-expression for functional strategies. RESULTS AND DISCUSSION Based on developmental defects, the observed phenotypes fell into several major classes, including defects in specific processes such as metamorphosis, formation of tail, head or nervous system (Table 1). We observed heterochronic changes of metamorphosis consisting, for instance, in an early, precocious reorganisation and rotation of the endoderm at twelve hours after hatching, not paralleled by tail resorption, or in the permanence of the larval stage three days after hatching. Another group of mutants consistently exhibited abnormalities in differentiation of tail structures, as the notochord which appeared shorter and wider when compared with the wild type. An interesting class comprises mutants with head defects ranging from the apical part of the head, to lack of the anterior portion of the endoderm or abnormal expansion of sensory organs and the neural vesicle. Another mutation lacked pigment in the otolith and ocellus, the two sensory cells of the neural vesicle (Fig. 2). These and other phenotypes will be described in detail elsewhere. Taken together, our preliminary results show that, in a manner similar to C. elegans, D. melanogaster and the zebrafish, it is possible to efficiently mutagenise ascidians and subsequently to identify mutants exhibiting a phenotype during larval development. The relatively small size of the ascidian genome and the possibility to conduct an F1 screen, indicate that it will be possible in the future to conduct larger scale mutagenesis screens, including maternal screens (Kimmel 1989), aiming to identify genes with a function in early development of the ascidian C. intestinalis. The genetic analysis of tunicates is therefore likely to provide substantial progress in understanding evolutionary modifications of developmental processes leading to the emergence of vertebrates. ACKNOWLEDGEMENTS This paper is dedicated to the memory of Prof. Nigel Holder. We thank W. Smith for sharing data prior to publication and S. Wilson for helpful comments on the manuscript. This research was funded by EMBO and EC (PS, CPH). Travel grants were provided by The Royal Society (CPH, PS) and The Company of Biologists (PS). Table 1. F2 developmental phenotypic classes identified using different ENU concentrations. Numbers in parentheses are percentages of potential carriers. Mutagen concentrations (mM ENU) 2.7 Potential carriers Lethal early phenotypes Multiple phenotypes Distinct phenotypes Head Metamorphosis Notochord/Tail Neural vescicle Ocellus Palps Gastrulation Wild-Type 24 14 (58) 4 (17) 9 (37) 2 0 1 1 0 1 0 1 1.35 14 9 (65) 1 (7) 4 (29) 2 1 0 0 0 0 0 1 0.27 24 6 (25) 7 (29) 17 (71) 1 2 1 3 1 0 1 2 176 Sarsia 85:173-176 – 2000 REFERENCES Chalfie M. 1993. Touch receptor development and function in Caenorhabditis elegans. Journal of neurobiology 24:1433-1441. Corbo JC, Levine M, Zeller RW. 1997. Characterization of a notochord-specific enhancer from the Brachyury promoter region of the ascidian, Ciona intestinalis. Development 124:589-602. Di Gregorio A, Levine M. 1998. Ascidian embryogenesis and the origins of the chordate body plan. Current Opinion in Genetics and Development 8:457-463. Driever W, Solnica-Krezel L, Schier AF, Neuhauss SCF, Malicki J, Stemple DL, Stainier DYR, Zwartkruis F, & al. 1996. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123:37-46. Gaiano N, Allende M, Amsterdam A, Kawakami K, Hopkins N. 1996. Highly efficient germ-line transmission of proviral insertions in zebrafish. Proceedings of the National Academy of Sciences USA 93:7777-7782. Glardon S, Holland LZ, Gehring WJ, Holland ND. 1998. Isolation and developmental expression of the amphioxus Pax-6 gene (AmphiPax-6): insights into eye and photoreceptor evolution. Development 125:2701-2710. Grunwald DJ, Streisinger G. 1992. Induction of recessive lethal and specific locus mutations in the zebrafish with ethyl nitrosourea. Genetics Research 59:103-116. Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van Eeden FJ, & al. 1996. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123:1-36. Hitotsumachi S, Carpenter DA, Russell WL. 1985. Dose-repetition increases the mutagenic effectiveness of N-ethylN-nitrosourea in mouse spermatogonia. Proceedings of the National Academy of Sciences USA 82:6619-6621. Jeffery WR, Swalla BJ. 1997. Tunicates. In: Gilbert SF, Raunio AM, editors. Embryology. Constructing the organism. Sinauer Ass. p 331-364. Kimmel CB. 1989. Genetics and early development of the zebrafish. Trends in Genetics 5:283-288. Lambert CC, Laird C. 1971. Molecular properties of tunicate DNA. Biochimica Biophysica Acta 240:39-45. Marino R, Pinto MR, Cotelli F, Lamia CL, De Santis R. 1998. The hsp70 protein is involved in the acquisition of gamete self-sterility in the ascidian Ciona intestinalis. Development 125:899-907. Mullins MC, Hammerschmidt M, Haffter P, Nüsslein-Volhard C. 1994. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Current Biology 4:189-202. Nicol DR, Meinertzhagen IA. 1988a. Development of the central nervous system of the larva of the ascidian, Ciona intestinalis L. 1. The early lineages of the neural plate. Developmental Biology 130:721-736. Nicol DR, Meinertzhagen IA. 1988b. Development of the central nervous system of the larva of the ascidian, Ciona intestinalis L. 2. Neural plate morphogenesis and cell lineages during neurulation. Developmental Biology 130:737-766. Nishida H. 1987. Cell lineage analysis in ascidian embryos by intracellular injection of a tracer enzyme. 3. Up to the tissue restricted stage. Developmental Biology 121:526541. Nüsslein-Volhard C, Wieschaus E. 1980. Mutations affecting segment number and polarity in Drosophila. Nature 287:795-801. Ohno S. 1970. Evolution by Gene Duplication. Heidelberg: Springer. 160 p. Postlethwaite JH, Yan YL, Gates MA, Horne S, Amores A, Brownlie A, Donovan A, Egan ES, & al. 1998. Vertebrate genome evolution and the zebrafish gene map. Nature Genetics 18:345-349. Riley BB, Grunwald DJ. 1995. Efficient induction of point mutations allowing recovery of specific locus mutations in zebrafish. Proceedings of the National Academy of Sciences USA 92:5997-6001. Russell WL, Kelly AM, Hunsicker PR, Bangham JW, Maddux SC, Phipps EL. 1979. Specific-locus test shows ethylnitrosourea to be the most potent mutagen in the mouse. Proceedings of the National Academy of Sciences USA 76:5818-5819. Satoh N. 1994. Developmental Biology of Ascidians. Cambridge, UK: Cambridge University Press. 234 p. Shimeld SM. 1997. Characterisation of amphioxus HNF-3 genes: conserved expression in the notochord and floor plate. Developmental Biology 183:74-85. Simmen M, Leitgeb S, Clark V, Jones S, Bird A. 1998. Gene number in an invertebrate chordate, Ciona intestinalis. Proceedings of the National Academy of Sciences USA 95:4437-4440. Solnica-Krezel L, Schier A, Driever W. 1994. Efficient recovery of ENU-induced mutations from the zebrafish germline. Genetics 136:1401-1420. Weinberg ES. 1998. Harnessing horizontal gene transfer. Current Biology 8:244-247. Westerfield M. 1993. The Zebrafish Book. Eugene, OR: University of Oregon Press. 392 p. Wittbrodt J, Meyer A, Schartl M. 1998. More genes in fish? BioEssays 20:511-515. Yoshikawa I, Ayaki T, Ohshima K. 1984. Comparative studies of dose-response curves for recessive lethal mutations induced by ethylnitrosourea in spermatogonia and spermatozoa of Drosophila melanogaster. Environmental Mutagenesis 6:489-496. Zimmering S, Thompson E. 1984. Comparison of rates of sexlinked recessive lethals induced by ethylnitrosourea (ENU) in post-meiotic cells of the male and oogonia of the female Drosophila. Environmental Mutagenesis 6:617-619. Accepted 11 January 2000 – Printed 9 June 2000 Editorial responsibility: Jarl Giske