* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 14 - Part I
Mitochondrial replacement therapy wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Lipid signaling wikipedia , lookup
Biosynthesis wikipedia , lookup
Magnesium transporter wikipedia , lookup
Magnesium in biology wikipedia , lookup
Signal transduction wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Photosynthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Western blot wikipedia , lookup
SNARE (protein) wikipedia , lookup
Microbial metabolism wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Mitochondrion wikipedia , lookup
Citric acid cycle wikipedia , lookup
Light-dependent reactions wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
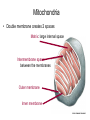
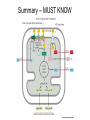
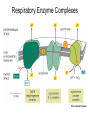
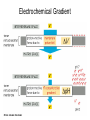
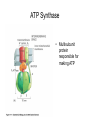
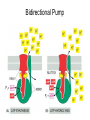
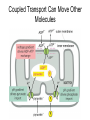
Chapter 14 Energy Generation in Mitochondria and Chloroplasts Generation of Energy • Millions of years ago there was no O2 available for oxidative phosphorylation to occur • Organisms produced energy from fermentation, still see this today • As O2 became available, a more efficient method of energy production developed – Based on the transfer of e- along the membrane Organism’s Energy Source • Small amount of ATP from glycolysis in the cytosol of cells • Majority made by a membrane based process in 2 stages – Stage 1 – e- transport chain • e- transferred along e- carriers in the membrane – Stage 2 – flow of H+ down an electrochemical gradient to produce ATP • Use a complex called ATP synthase Stage 1 • NADH (from the Kreb’s cycle) brings in the e- and transfers them to the carrier molecules • The e- moves down the chain and looses energy at each step – as this happens, H+ are pumped across the membrane • This creates an electro-chemical gradient across the membrane Stage 2 • The electrochemical gradient is a form of stored energy – it has the potential to do work • The H+ can now move down the gradient and return to the other side of the membrane thru ATP synthase – in this process, generates ATP from ADP and Pi Chemiosmotic Coupling • Once called the chemiosmotic hypothesis – Chemi from making ATP, osmotic because of crossing the membrane • Now known as chemiosmotic coupling Mitochondria • Produce most of a cells ATP – acetyl groups in the Kreb’s cycle producing CO2 and NADH • NADH donates the e- to the electron transport chain and becomes oxidized to NAD+ • e- transfer promotes proton pump and ATP synthesis in process called oxidative phosphorylation • Cells that require large amounts of energy such as the heart have large numbers of mitochondria Mitochondria • Contain their own copies of DNA and RNA along with transcription and translation system (ribosomes) • Are able to regenerate themselves without the whole cell undergoing division • Shape and size dependent on what the cell’s function is Mitochondria • Double membrane creates 2 spaces Matrix: large internal space Intermembrane space: between the membranes Outer membrane Inner membrane Mitochondria Inner Membrane • Inner membrane is the site of the e- transport chain, across which the proton pump occurs and contains ATP synthase • Inner membrane is highly folded – called cristae – increasing the surface area on which the above reactions can take place High Energy e• Mitochondria use pyruvate and fatty acids and convert it to acetyl CoA in the matrix • Citric acid cycle generates NADH and FADH2 which carry the e- to the electron transport chain Summary – MUST KNOW Proton Pumping • Many molecules can supply the e- - carbohydrates and fatty acids • O2 ultimate e- acceptor producing H2O as waste Movement of Electrons Oxidative Phosphorylation Electron Transport Chain • Resides in the inner mitochondrial membrane – also called respiratory chain • 15 proteins involved in the chain – grouped in 3 large respiratory enzyme complexes – NADH dehydrogenase complex – Cytochrome b-c1 complex – Cytochrome oxidase complex • Pumps protons across the membrane as e- are transferred thru them Respiratory Enzyme Complexes Proton Gradient • e- transfer is an oxidation/reduction reaction • NADH has high-energy e- has a low electron affinity so the e- is readily passed to NADH dehydrogenase and so on down the chain • Each transfer couples the energy released with the uptake of a H+ from the matrix to the intermembrane space setting up the electrochemical gradient Proton Gradient • Gradient of proton (H+) concentration across the inner mitochondrial membrane – a pH gradient with the pH in the matrix higher than in the intermembrane space • Proton pumping also generates a membrane potential – matrix side is negative and intermembrane space is positive 4 Complexes in Membrane Location of H+ Electrochemical Gradient Oxidative Phosphorylation • ATP synthase is the protein complex responsible for making ATP by creating a path for H+ thru the membrane • ATP synthase is an enzyme ATP Synthase • Multisubunit protein responsible for making ATP Summary Bidirectional Pump Coupled Transport Can Move Other Molecules Oxidation of Sugar and Fats