* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 9 Powerpoint
Metalloprotein wikipedia , lookup
Mitochondrion wikipedia , lookup
Butyric acid wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthesis wikipedia , lookup
Phosphorylation wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Electron transport chain wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Microbial metabolism wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biochemistry wikipedia , lookup
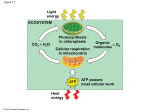
Chapter 9 Cellular Respiration: Harvesting Chemical Energy PowerPoint® Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings • Energy flows into an ecosystem as sunlight and leaves as heat • Photosynthesis generates O2 and organic molecules, which are used in cellular respiration • Cells use chemical energy stored in organic molecules to regenerate ATP, which powers work Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-2 Light energy ECOSYSTEM Photosynthesis in chloroplasts CO2 + H2O Organic +O molecules 2 Cellular respiration in mitochondria ATP ATP powers most cellular work Heat energy • Organic compounds possess potential energy as a result of their arrangement of atoms. • With the help of enzymes, a cell systematically degrades complex organic molecules that are rich in potential energy to simpler waste products that have less energy. • Some of the energy taken out of chemical storage can be used to do work; the rest is dissipated as heat. 1. Distinguish between: • The breakdown of organic molecules is exergonic • Fermentation is a partial degradation of sugars that occurs without O2 • Aerobic respiration (most prevalent) consumes organic molecules and O2 and yields ATP • Anaerobic respiration is similar to aerobic respiration but consumes compounds other than O2 • Cellular respiration includes both aerobic and anaerobic respiration but is often used to refer to aerobic respiration Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings 2. Write down the formula for the breakdown of glucose in cellular respiration. • Although carbohydrates, fats, and proteins are all consumed as fuel, it is helpful to trace cellular respiration with the sugar glucose: C6H12O6 + 6 O2 6 CO2 + 6 H2O + Energy (ATP + heat) Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings 3. How do the catabolic pathways that decompose glucose and other organic fuels yield energy? • The relocation of electrons during the chemical reactions of respiration releases energy stored in organic molecules, and this energy ultimately is used to synthesize ATP. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings 4. What is a redox reaction? Define the following: • Chemical reactions that transfer electrons between reactants are called oxidation-reduction reactions, or redox reactions • In oxidation, a substance loses electrons, or is oxidized • In reduction, a substance gains electrons, or is reduced (the amount of positive charge is reduced) Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings • The electron donor is called the reducing agent • The electron receptor is called the oxidizing agent • Some redox reactions do not transfer electrons but change the electron sharing in covalent bonds • An example is the reaction between methane and O2 Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-UN1 becomes oxidized (loses electron) becomes reduced (gains electron) Fig. 9-UN2 becomes oxidized becomes reduced Fig. 9-3 Reactants Products becomes oxidized becomes reduced Methane (reducing agent) Oxygen (oxidizing agent) Carbon dioxide Water • An electron loses potential energy when it shifts from a less electronegative atom toward a more electronegative one, just like a ball rolling downhill. • A redox reaction that moves electrons closer to oxygen, such as the burning of methane, therefore releases chemical energy that can be put to work. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings 5. In general, why are organic molecules that have an abundance of hydrogen atoms excellent fuels? • They are excellent fuels because their bonds are a source of “hilltop” electrons. • Their energy may be released as these electrons “fall” down an energy gradient when they are transferred to oxygen. • In respiration, the oxidation of glucose transfers electrons to a lower energy state, liberating energy that becomes available for ATP synthesis. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings 6. Describe the difference between igniting glucose and burning it, and how we break it down in our bodies. • Cellular respiration does not oxidize glucose in a single explosive step. • Rather, we breakdown glucose in a step-bystep process, releasing energy in small increments rather than in one large quantity. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings 7. What is NAD+? What does it become when it is reduced? What is its role in cellular respiration? • In cellular respiration, glucose and other organic molecules are broken down in a series of steps • Electrons from organic compounds are usually first transferred to NAD+, a coenzyme • As an electron acceptor, NAD+ functions as an oxidizing agent during cellular respiration • Each NADH (the reduced form of NAD+) represents stored energy that is tapped to synthesize ATP Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-4 2 e– + 2 H+ 2 e– + H+ NADH H+ Dehydrogenase Reduction of NAD+ NAD+ + + H+ 2[H] Oxidation of NADH Nicotinamide (reduced form) Nicotinamide (oxidized form) 8. What is an electron transport chain? Why is it so important in regards to the release of energy? • NADH passes the electrons to the electron transport chain • Unlike an uncontrolled reaction, the electron transport chain passes electrons in a series of steps instead of one explosive reaction • O2 pulls electrons down the chain in an energyyielding tumble • The energy yielded is used to regenerate ATP Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-5 H2 + 1/2 O2 2H (from food via NADH) Controlled release of + – 2H + 2e energy for synthesis of ATP 1/ 2 O2 Explosive release of heat and light energy 1/ (a) Uncontrolled reaction (b) Cellular respiration 2 O2 9. What element captures electrons at the bottom of the ETC. What is formed? • Oxygen 10. In summary, during cellular respiration, most electrons travel the following “downhill” route: glucose NADH ETC oxygen 11. Briefly list and describe the three stages of cellular respiration. • Cellular respiration has three stages: – Glycolysis (breaks down glucose into two molecules of pyruvate) – The citric acid cycle (completes the breakdown of glucose) – Oxidative phosphorylation (accounts for most of the ATP synthesis) For each molecule of glucose, between about 3638 ATP, although 36 is most widely used. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-6-1 Electrons carried via NADH Glycolysis Pyruvate Glucose Cytosol ATP Substrate-level phosphorylation Fig. 9-6-2 Electrons carried via NADH and FADH2 Electrons carried via NADH Citric acid cycle Glycolysis Pyruvate Glucose Mitochondrion Cytosol ATP ATP Substrate-level phosphorylation Substrate-level phosphorylation Fig. 9-6-3 Electrons carried via NADH and FADH2 Electrons carried via NADH Citric acid cycle Glycolysis Pyruvate Glucose Oxidative phosphorylation: electron transport and chemiosmosis Mitochondrion Cytosol ATP ATP ATP Substrate-level phosphorylation Substrate-level phosphorylation Oxidative phosphorylation • The process that generates most of the ATP is called oxidative phosphorylation because it is powered by redox reactions • Oxidative phosphorylation accounts for almost 90% of the ATP generated by cellular respiration BioFlix: Cellular Respiration Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings • A smaller amount of ATP is formed in glycolysis and the citric acid cycle by substrate-level phosphorylation Enzyme Enzyme ADP P Substrate + Product ATP 13. What does the word “glycolysis” mean? • Glycolysis (“splitting of sugar”) breaks down glucose into two molecules of pyruvate • Glycolysis occurs in the cytoplasm and has two major phases: – Energy investment phase – Energy payoff phase Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings 14. List and describe the two phases of glycolysis. • Glycolysis occurs in the cytoplasm and has two major phases: – Energy investment phase • The cell spends 2 ATP to get the process rolling – Energy payoff phase • ATP is produced during substrate-level phosphorylation • NAD+ is reduced to NADH by electrons released from the oxidation of glucose Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-8 Energy investment phase Glucose 2 ADP + 2 P 2 ATP used 4 ATP formed Energy payoff phase 4 ADP + 4 P 2 NAD+ + 4 e– + 4 H+ 2 NADH + 2 H+ 2 Pyruvate + 2 H2O Net Glucose 4 ATP formed – 2 ATP used 2 NAD+ + 4 e– + 4 H+ 2 Pyruvate + 2 H2O 2 ATP 2 NADH + 2 H+ 15. In the end, where has all the carbon from glucose gone? • In the end, all of the carbon originally present in glucose is accounted for in the two molecules of pyruvate. • No CO2 is released during glycolysis. • Glycolysis occurs whether or not O2 is present • If O2 is present, the chemical energy stored in pyruvate and NADH can be extracted by the citric acid cycle and oxidative phosphorylation. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Question 16 • 16. Glycolysis releases less than a quarter of the chemical energy stored in glucose; most of the energy remains stockpiled in the two molecules of pyruvate. If molecular oxygen is present, the pyruvate enters the mitochondria, where the enzymes of the citric acid cycle complete the oxidation of glucose. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Concept 9.3: The citric acid cycle completes the energy-yielding oxidation of organic molecules • In the presence of O2, pyruvate enters the mitochondrion • Before the citric acid cycle can begin, pyruvate must be converted to acetyl CoA, which links the cycle to glycolysis Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-10 CYTOSOL MITOCHONDRION NAD+ NADH + H+ 2 1 Pyruvate Transport protein 3 CO2 Coenzyme A Acetyl CoA 17. For each acetyl group entering the cycle, please tally the number of NADH, FADH, and ATP molecules that are produce. • The citric acid cycle, also called the Krebs cycle, takes place within the mitochondrial matrix • The cycle oxidizes organic fuel derived from pyruvate, generating 1 ATP, 3 NADH, and 1 FADH2 per turn Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-11 Pyruvate CO2 NAD+ CoA NADH + H+ Acetyl CoA CoA CoA Citric acid cycle FADH2 2 CO2 3 NAD+ 3 NADH FAD + 3 H+ ADP + P i ATP • The citric acid cycle has eight steps, each catalyzed by a specific enzyme • The acetyl group of acetyl CoA joins the cycle by combining with oxaloacetate, forming citrate • The next seven steps decompose the citrate back to oxaloacetate, making the process a cycle • The NADH and FADH2 produced by the cycle relay electrons extracted from food to the electron transport chain Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-12-1 Acetyl CoA CoA—SH 1 Oxaloacetate Citrate Citric acid cycle Fig. 9-12-2 Acetyl CoA CoA—SH H2O 1 Oxaloacetate 2 Citrate Isocitrate Citric acid cycle Fig. 9-12-3 Acetyl CoA CoA—SH 1 H2O Oxaloacetate 2 Citrate Isocitrate NAD+ Citric acid cycle 3 NADH + H+ CO2 -Ketoglutarate Fig. 9-12-4 Acetyl CoA CoA—SH 1 H2O Oxaloacetate 2 Citrate Isocitrate NAD+ Citric acid cycle NADH + H+ 3 CO2 CoA—SH -Ketoglutarate 4 NAD+ Succinyl CoA NADH + H+ CO2 Fig. 9-12-5 Acetyl CoA CoA—SH 1 H2O Oxaloacetate 2 Citrate Isocitrate NAD+ Citric acid cycle NADH + H+ 3 CO2 CoA—SH -Ketoglutarate 4 CoA—SH 5 NAD+ Succinate GTP GDP ADP ATP Pi Succinyl CoA NADH + H+ CO2 Fig. 9-12-6 Acetyl CoA CoA—SH H2O 1 Oxaloacetate 2 Citrate Isocitrate NAD+ Citric acid cycle NADH + H+ 3 CO2 Fumarate CoA—SH 6 -Ketoglutarate 4 CoA—SH 5 FADH2 NAD+ FAD Succinate GTP GDP ADP ATP Pi Succinyl CoA NADH + H+ CO2 Fig. 9-12-7 Acetyl CoA CoA—SH H2O 1 Oxaloacetate 2 Malate Citrate Isocitrate NAD+ Citric acid cycle 7 H2O NADH + H+ 3 CO2 Fumarate CoA—SH -Ketoglutarate 4 6 CoA—SH 5 FADH2 NAD+ FAD Succinate GTP GDP ADP ATP Pi Succinyl CoA NADH + H+ CO2 Fig. 9-12-8 Acetyl CoA CoA—SH NADH +H+ H2O 1 NAD+ 8 Oxaloacetate 2 Malate Citrate Isocitrate NAD+ Citric acid cycle 7 H2O NADH + H+ 3 CO2 Fumarate CoA—SH 6 -Ketoglutarate 4 CoA—SH 5 FADH2 NAD+ FAD Succinate GTP GDP ADP ATP Pi Succinyl CoA NADH + H+ CO2 Concept 9.4: During oxidative phosphorylation, chemiosmosis couples electron transport to ATP synthesis • Following glycolysis and the citric acid cycle, NADH and FADH2 account for most of the energy extracted from food • These two electron carriers donate electrons to the electron transport chain, which powers ATP synthesis via oxidative phosphorylation Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Question 18 • Glycolysis and the citric acid cycle produce only 4 ATP molecules per glucose molecule; 2 net ATP from glycolysis and 2 ATP from the citric acid cycle. At this point, molecules of NADH and FADH2 account for most of the energy extracted from the glucose. The electron escorts link glycolysis and the citric acid cycle to the machinery of oxidative phosphorylation, which uses energy released from the electron transport chain to power ATP synthesis. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings The Pathway of Electron Transport • The electron transport chain is in the cristae of the mitochondrion • Most of the chain’s components are proteins, which exist in multiprotein complexes • The carriers alternate reduced and oxidized states as they accept and donate electrons • Electrons drop in free energy as they go down the chain and are finally passed to O2, forming H2O • Electrons are transferred from NADH or FADH2 to the electron transport chain • The electron transport chain generates no ATP • The chain’s function is to break the large free-energy drop from food to O2 into smaller steps that release energy in manageable amounts Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-13 NADH 50 2 e– NAD+ FADH2 2 e– 40 FMN FAD Multiprotein complexes FAD Fe•S Fe•S Q Cyt b 30 Fe•S Cyt c1 I V Cyt c Cyt a Cyt a3 20 10 2 e– (from NADH or FADH2) 0 2 H+ + 1/2 O2 H2O 19. Define the following: • ATP synthase – a protein complex (enzyme) that makes ATP from ADP and inorganic phosphate • Chemiosmosis – the process in which energy stored in the form of a hydrogen ion gradient across a membrane is used to drive cellular work such as the synthesis of ATP. • Proton-motive force – the H+ gradient formed across a membrane that is capable of performing work Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-14 INTERMEMBRANE SPACE H+ Stator Rotor Internal rod Catalytic knob ADP + P i ATP MITOCHONDRIAL MATRIX Fig. 9-15 EXPERIMENT Magnetic bead Electromagnet Sample Internal rod Catalytic knob Nickel plate RESULTS Rotation in one direction Number of photons detected (103) Rotation in opposite direction No rotation 30 25 20 0 Sequential trials Fig. 9-15a EXPERIMENT Magnetic bead Electromagnet Sample Internal rod Catalytic knob Nickel plate Fig. 9-15b RESULTS Rotation in one direction Rotation in opposite direction No rotation 30 25 20 0 Sequential trials Fig. 9-16 H+ H+ H+ H+ Protein complex of electron carriers Cyt c V Q ATP synthase FADH2 NADH 2 H+ + 1/2O2 H2O FAD NAD+ ADP + P i (carrying electrons from food) ATP H+ 1 Electron transport chain Oxidative phosphorylation 2 Chemiosmosis Question 21 • During cellular respiration, most energy flows in this sequence: glucose NADH electron transport chain proton-motive force ATP • About 40% of the energy in a glucose molecule is transferred to ATP during cellular respiration, making about 38 ATP Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-17 Electron shuttles span membrane CYTOSOL 2 NADH Glycolysis Glucose 2 Pyruvate MITOCHONDRION 2 NADH or 2 FADH2 6 NADH 2 NADH 2 Acetyl CoA + 2 ATP Citric acid cycle + 2 ATP Maximum per glucose: About 36 or 38 ATP 2 FADH2 Oxidative phosphorylation: electron transport and chemiosmosis + about 32 or 34 ATP 24. What are the two mechanisms cells can use to generate ATP when oxygen is not present? • In the absence of O2, glycolysis couples with fermentation or anaerobic respiration to produce ATP • The distinction between these two is based on whether an ETC is present. • Anaerobic respiration uses an electron transport chain with an electron acceptor other than O2, for example sulfate • Fermentation uses phosphorylation instead of an electron transport chain to generate ATP Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings 25. Fermentation is a way of harvesting chemical energy without using either oxygen or any electron transport chain. • In other words, without cellular respiration. 26. As an alternative to respiratory oxidation or organic nutrients, fermentation is an expansion of glycolysis that allows continuous generation of ATP by the substrate-level phosphorylation of glycolysis. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings 27. Briefly describe the following two processes” • In alcohol fermentation, pyruvate is converted to ethanol in two steps – The first step releases carbon dioxide from the pyruvate, which is converted to the two-carbon compound acetaldehyde – In the second step, acetaldehyde is reduced by NADH to ethanol, regenerating the supply of NAD+ • Alcohol fermentation by yeast is used in brewing, winemaking, and baking Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-18a 2 ADP + 2 P i Glucose 2 ATP Glycolysis 2 Pyruvate 2 NAD+ 2 Ethanol (a) Alcohol fermentation 2 NADH + 2 H+ 2 CO2 2 Acetaldehyde • In lactic acid fermentation, pyruvate is reduced to NADH, forming lactate as an end product, with no release of CO2 • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt • Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce • It was thought that lactic acid caused muscle soreness, but recent research suggests that it might be increased levels of potassium ions. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-18b 2 ADP + 2 P i Glucose 2 ATP Glycolysis 2 NAD+ 2 NADH + 2 H+ 2 Pyruvate 2 Lactate (b) Lactic acid fermentation Fermentation and Aerobic Respiration Compared • Both processes use glycolysis to oxidize glucose and other organic fuels to pyruvate • The processes have different final electron acceptors: an organic molecule (such as pyruvate or acetaldehyde) in fermentation and O2 in cellular respiration • Cellular respiration produces 38 ATP per glucose molecule; fermentation produces 2 ATP per glucose molecule Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings 29. What is the difference between obligate anaerobes and facultative anaerobes?. • Obligate anaerobes carry out fermentation or anaerobic respiration and cannot survive in the presence of O2 • Yeast and many bacteria are facultative anaerobes, meaning that they can survive using either fermentation or cellular respiration • In a facultative anaerobe, pyruvate is a fork in the metabolic road that leads to two alternative catabolic routes Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-19 Glucose CYTOSOL Glycolysis Pyruvate No O2 present: Fermentation O2 present: Aerobic cellular respiration MITOCHONDRION Ethanol or lactate Acetyl CoA Citric acid cycle 30. What is the evolutionary significance of glycolysis? • Glycolysis occurs in nearly all organisms • Glycolysis probably evolved in ancient prokaryotes before there was oxygen in the atmosphere Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Concept 9.6: Glycolysis and the citric acid cycle connect to many other metabolic pathways • Gycolysis and the citric acid cycle are major intersections to various catabolic and anabolic pathways Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings The Versatility of Catabolism • Catabolic pathways funnel electrons from many kinds of organic molecules into cellular respiration • Glycolysis accepts a wide range of carbohydrates • Proteins must be digested to amino acids; amino groups can feed glycolysis or the citric acid cycle Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings • Fats are digested to glycerol (used in glycolysis) and fatty acids (used in generating acetyl CoA) • Fatty acids are broken down by beta oxidation and yield acetyl CoA • An oxidized gram of fat produces more than twice as much ATP as an oxidized gram of carbohydrate Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-20 Proteins Carbohydrates Amino acids Sugars Glycolysis Glucose Glyceraldehyde-3- P NH3 Pyruvate Acetyl CoA Citric acid cycle Oxidative phosphorylation Fats Glycerol Fatty acids Regulation of Cellular Respiration via Feedback Mechanisms • Feedback inhibition is the most common mechanism for control • If ATP concentration begins to drop, respiration speeds up; when there is plenty of ATP, respiration slows down • Control of catabolism is based mainly on regulating the activity of enzymes at strategic points in the catabolic pathway Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings You should now be able to: 1. Explain in general terms how redox reactions are involved in energy exchanges 2. Name the three stages of cellular respiration; for each, state the region of the eukaryotic cell where it occurs and the products that result 3. In general terms, explain the role of the electron transport chain in cellular respiration Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings 4. Explain where and how the respiratory electron transport chain creates a proton gradient 5. Distinguish between fermentation and anaerobic respiration 6. Distinguish between obligate and facultative anaerobes Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings