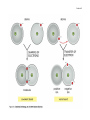
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electron - CoolHub
Oxidation state wikipedia , lookup
Photoelectric effect wikipedia , lookup
Molecular orbital wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Nuclear binding energy wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Bent's rule wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Livermorium wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Elementary particle wikipedia , lookup
Condensed matter physics wikipedia , lookup
Chemical element wikipedia , lookup
Hydrogen atom wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Bond valence method wikipedia , lookup
History of chemistry wikipedia , lookup
Electronegativity wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Periodic table wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Atomic orbital wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Metallic bonding wikipedia , lookup
Extended periodic table wikipedia , lookup
History of molecular theory wikipedia , lookup
Chemical bond wikipedia , lookup
Electron configuration wikipedia , lookup
Lesson 1 Standard: MS-PS1 Matter and Its Interactions PE: MS-PS1-1 Develop models to describe the atomic composition of simple molecules and extended structures. Practice: Disciplinary Core Idea: Developing and Using Models Develop a model to predict and/or describe phenomena. Develop a model to describe unobservable mechanisms. Objectives Students will be able to build a model to demonstrate aspects of atomic structure PS1.A: Structure and Properties of Matter Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms. Classroom Activities Students will begin recalling information they learned in sixth grade be taking a pretest. This pre-test is 10 questions that comes from their mid unit test. Once the test is over they will begin the lab “Investigating the Size of Atomic Particles.” I have done this lab two different ways. If the lab is done as is, the students will have to go outside to measure the full distance between the fake proton and electron. For rainy days, I have had the students each hold onto a piece of the long 70m string of yarn. This stretches the length of the hallway. The formula for the calculation of the atomic radius is 50000m*diameter of proton Crosscutting Concept: Scale, Proportion, and Quantity Time, space, and energy phenomena can be observed at various scales using models to study systems that are too large or too small. Assessments Students will be assessed with a pre-test for the beginning of the unit. Students will have to complete the lab activity questions. Lesson 1 Chemical Interactions Pre-Unit 1. What are the three parts of an atom and how are they related to each other? 2. Label the parts of an element shown below from the 2 periodic table. He 4.0026 3. As the atomic number, or number of protons, increases in the periodic table, what happens to the number of electrons? Why? 4. What is the difference between and ionic and covalent bond? 5. How do you know whether a reaction is endothermic or exothermic? Lesson 1 Quick Lab Investigate the Size of Atomic Particles In this lab, you will build a scale model of a hydrogen atom. Your teacher will provide information regarding the relative size of the proton compared with the relative distance of an electron from a proton in a hydrogen atom. PROCEDURE Draw a small circle about 1 millimeter (mm) (0.1 centimeter [cm]) in diameter in the space below. Measure and record the diameter of the circle. ____________________________________________________ Assuming the diameter of the circle represents a proton, calculate the relative distance of the electron from the proton in a hydrogen atom. Show your calculation in the space below. OBJECTIVE • Build a model to demonstrate aspects of atomic structure. MATERIALS For each pair of students • calculator • meterstick • peas, dried • pencil • ruler, metric • scissors • yarn, 70 m Roll out a length of yarn to reflect the length you just calculated; this will illustrate the relative distance of the electron from the proton. What was the length of yarn you used? _________________________________________________________________________ What can you conclude when comparing the size of the proton with the distance of the electron from the proton? _________________________________________________________________________ _________________________________________________________________________ Measure the diameter of a pea with the metric ruler and record this value. _________________________________________________________________________ Lesson 1 Quick Lab continued Assuming the diameter of the pea represents a proton, calculate the relative distance of the electron from the proton in a hydrogen atom. What do you think lies between the proton and the electron in a hydrogen atom? _________________________________________________________________________ _________________________________________________________________________ Is the electron always in one spot in a hydrogen atom, or does its position change? _________________________________________________________________________ _________________________________________________________________________ Lesson 2 Standard: Standard: MS-PS1 Matter and Its Interactions PE: MS-PS1-1 Develop models to describe the atomic composition of simple molecules and extended structures. Practice: Disciplinary Core Idea: Obtaining, Evaluating, and Communicating Information Objectives Discuss the atomic particles of an atom. Create a model of the parts of the Atomic Theory Explain the parts of the atomic theory and how it relates to the way we view atoms today. PS1.A: Structure and Properties of Matter Classroom Activities Crosscutting Concept: Interdependence of Science, Engineering, and Technology Assessments The lesson starts out with a power point slide review of the Students are assessed based parts of the atom discussed on a rubric. Students in class from the previous day. are required to complete a worksheet created by the Students are then broken into group. groups for Atomic Theory review. Students are to read about atomic theory form their books (pg 8-11). Theories include Dalton, Thompson, Rutherford, Bohr, Cloud Model and Modern Theory. Each group is responsible to presenting their assigned atomic theory to the class (see attached for presentation criteria) Lesson 2 Atomic Particles • Protons, Electrons, Neutrons • Atomic Particles are TINY!!! • Protons=positive charge • Electrons=negative charge • Electrons are VERY far away from the Protons • Electrons are attracted to the proton but move too fast to attach to it Lesson 2 Atomic Particles • Protons, Electrons, Neutrons • Atomic Particles are ____________!!! • Protons=_______________ charge • Electrons=______________ charge • Electrons are VERY far away from the Protons • Electrons are _________ to the proton but move too ___________ to attach to it Lesson 2 Group Work • Read in your book about your part of the Atomic Theory • Draw a model of your assigned atomic theory to present to the class. • Create a worksheet for the class to complete about your theory. • Duties for presentation. Each student in your group must do 1 duty. – One person talks about the person that discovered the theory – One person talks about what the theory is about – One person describes the model and what the model reminds them of – One person describes why this theory is no longer acceptable (or if the modern theory, why it is the accepted theory) – One person explains the worksheet page. Lesson 3 Standard: MS-PS1 Matter and Its Interactions PE: MS-PS1- Develop models to describe the atomic composition of simple molecules and extended structures MS-PS3 Gather and make sense of information to describe how synthetic materials come from natural resources and impact society Practice: Disciplinary Core Idea: Crosscutting Concept: Developing and Using Models PS1.A: Structure and Properties of Matter Structure and Function Analyzing and Interpreting Data Patterns Scale, Proportion, and Quantity Objectives Classroom Activities Assessments Powerpoint Presentation with fill in the blank notes Students will be able to define the parts of the periodic table and identify patters in the in the structure of the table. Students will be able to locate an element based on a given property. Students will graph values of atomic radaii and interpret trends in the periodic table Build a model of the first 20 elements on the periodic table based on properties given on cards. Found at www.middleschoolchemistry.c om This is Chapter 4, Lesson 2. The activity itself is included here but you have to go to the website for the cards and student worksheets. Recognizing patterns Lab Graph the atomic radaii of the periods and groups on the periodic table. (Predicting Properties Lab and Comparing Atom Sizes Lab) Watch “Hunting the Element” a PBS special on how elements behave and how we use elements on a regular basis. Post Lab Questions in Predicting Properties Lab Analyze and Conclude Questions in Comparing Atomic Sizes Lab Exit Tickets Write a paper on how synthetic glass is made, how we use synthetic glass in our lives, and the benefit of synthetic glass. Lesson 3 The Periodic Table! Lesson 3 What is it? • Used worldwide • Organizes elements into categories • Based on the number of valence electrons Lesson 3 What is a valence electron? • Have the highest energy level • Electrons held most loosely by proton (furthest from nucleus) • Determines the properties of the element • Determines which atoms can bond (or combine) together Lesson 3 Information in the Periodic Table • Atomic Symbol • Atomic Number • Period • Group/Family • Atomic Mass Lesson 3 Atomic Symbol • One or Two letters • Identifies the element • BIG LETTERS • Name is normally listed underneath Lesson 3 Atomic Number • The number of protons in the nucleus • Numbers increase in the periodic table Lesson 3 Period • Rows of elements in the periodic table • Number is normally listed to the left of the row • Atomic Number increases across the period • Number of electrons increases one at a time across the period Lesson 3 Group/Family • Elements in the same column • Group numbers are listed at the top of the column (many times in roman numerals) • Always have the same number of valence electrons Lesson 3 Atomic Mass • Atomic mass of an element’s atoms • This includes Protons, Neutrons, and Electrons Lesson 3 Let’s focus on the Groups • Noble Gases • Alkali Metals • Halogens • Metals • Nonmetals • Metalloids Lesson 3 Noble Gases • Group 18 • Have 8 valence electrons • Very very Stable • Do not react with other elements Lesson 3 Alkali Metals • Group 1 • Have 1 valence electron • Most of the time Share the electron to other elements in bonding • Very very Reactive Lesson 3 Halogens • Group 17 • Have 7 Valence electrons • Like to gain an electron to be stable • Bonds with elements that like to share valence electrons Lesson 3 Metals • Groups 2-12 • Have 1, 2, or 3 valence electrons • Most like to lose valence electrons in bonding • Can be very reactive Lesson 3 Non-Metals • Have 4 or more valence electrons • Usually combine with metals • Like to gain electrons in bonding • Flourine is most reactive non-metal Lesson 3 Metalloids • Have 3-6 valence electrons • Gain or lose electrons during bonding • Can act as a metal or non-metal Lesson 3 Lesson 3 Lesson 3 Lesson 3 Chapter 4, Lesson 2: The Periodic Table Key Concepts The periodic table is a chart c • ontaining information about the atoms that make up all matter. • An element is a substance made up of only one type of atom. • The atomic number of an atom is equal to the number of protons in its nucleus. • The number of electrons surrounding the nucleus of an atom is equal to the number of protons in its nucleus. • Different atoms of the same element can have a different number of neutrons. • Atoms of the same element with different numbers of neutrons are called “isotopes” of that element. • The atomic mass of an element is the average mass of the different isotopes of the element. • The atoms in the periodic table are arranged to show characteristics and relationships between atoms and groups of atoms. Summary Students will begin to look closely at the periodic table. They will be introduced to the basic information given for the elements in most periodic tables: the name, symbol, atomic number, and atomic mass for each element. Students will focus on the first 20 elements. They will try to correctly match cards with information about an element to each of the first 20 elements. Students will then watch several videos of some interesting chemical reactions involving some of these elements. Objective Students will identify different atoms by the number of protons in the nucleus and realize that the number of electrons equals the number of protons in a neutral atom. They will also be able to explain the meaning of atomic number and atomic mass. Evaluation The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan. The activity sheets are formative assessments of student progress and understanding. Lesson 3 About this Lesson Lessons 2 and 3 both use the 20 atom description cards beginning on page 240. ©2011 American Chemical Society Middle School Chemistry Unit 259 Teacher preparation Print out the 20 pages of element cards. The first page is shown. Laminate each page and cut out the cards. For Lesson 2, you will need the 5 cards for each element from the left side of each sheet. You will also need the card in the upper right corner. This is the atom name card. Tape each of the 20 atom name cards to a spot in the room where students can place the cards that match that atom nearby. For Lesson 3, you will need the atom name card, taped in the same location in the room, and the four cards beneath it. Divide the class into 10 groups of 2 or 3 students each. ENGAGE 1. Introduce students to the periodic table. Project the image Periodic Table. www.middleschoolchemistry.com/multimedia/chapter4/lesson2#periodic_table Tell students that this is the periodic table. Explain that each box contains information about a different atom. The periodic table shows all the atoms that everything in the known universe is made from. It’s kind of like the alphabet in which only 26 letters, in different combinations, make up many thousands of words. The 100 or so atoms of the periodic table, in different combinations, make up millions of different substances. Note: It is often confusing for students to see the terms “atom” and “element” used interchangeably as if they are the same thing. Explain to students that an atom is the smallest particle or “building block” of a substance. An element is a substance made up of all the same type of atom. For instance, a piece of pure carbon is made up of only carbon atoms. This piece of pure carbon is a sample of the element carbon. The people who developed the periodic table could have called it the Periodic Table of the Atoms but they did not have a firm understanding of atoms at that time. Since they were working with actual samples of elements such as copper, mercury, sulfur, etc., they called it the periodic table of the elements. Optional Play one or both of the following songs. • The Elements by Tom Lehrer with animation by Mike Stanfill Lesson 3 www.privatehand.com/flash/elements.html • Meet the Elements by They Might be Giants www.youtube.com/watch?v=d0zION8xjbM 2. Explain the meaning of the numbers and letters in the boxes in the periodic table. Tell students that the class will focus on the first 20 elements over 2 days. On the first day, they will look at the number of protons, electrons, and neutrons in the atoms of each element. On the second day, they will look at the arrangement of electrons in the atoms. Give each student a copy of the periodic table of the elements, the periodic table of elements 1–20, and the activity sheet. Students will use the periodic table of elements 1–20, along with the activity sheet, in the lesson they will do today. Explain what the numbers and letters in each box on the periodic table represent. Explain atomic mass. The atomic mass of an element is based on the mass of the protons, neutrons, and electrons of the atoms of that element. The mass of the proton and neutron are about the same, but the mass of the electron is much smaller (about 1/2000 the mass of the proton or neutron). The majority of the atomic mass is contributed by the protons and neutrons. For any element in the periodic table, the number of electrons in an atom of that element always equals the number of protons in the nucleus. But this is not true for neutrons. Atoms of the same element can have different numbers of neutrons than protons. Atoms of the same element with different numbers of neutrons are called isotopes of that element. The atomic mass in the periodic table is an average of the atomic mass of the isotopes of an element. For the atoms of the first 20 elements, the number of neutrons is either equal Lesson 3 to or slightly greater than the number of protons. For example, the vast majority of carbon atoms have 6 protons and 6 neutrons, but a small percentage have 6 protons and 7 neutrons, and an even smaller percentage have 6 protons and 8 neutrons. Since the majority of carbon atoms have a mass very close to 12, and only a small percentage are greater than 12, the average atomic mass is slightly greater than 12. 3. Describe the activity students will do to learn about the first 20 elements of the periodic table. Show students that you have 100 cards (5 for each of the first 20 elements). Explain that each card contains information about one of the first 20 atoms of the periodic table. The students’ job is to read the card carefully, figure out which atom the card is describing, and put the card at the spot in the room for that atom. Review the information about protons, electrons, and neutrons students need to know in order to match the cards with the correct element: Proton • Positively charged particle in the nucleus of the atom. • The number of protons in an atom’s nucleus is the atomic number. Electron • Negatively charged particle surrounding the nucleus of the atom. • The number of electrons surrounding the nucleus of an atom is equal to the number of protons in the atom’s nucleus. Neutron Particle in the n • ucleus that has almost the same mass as a proton but has no charge. • For the atoms of the first 20 elements, the number of neutrons is either equal to or slightly greater than the number of protons. To match the number of neutrons listed on your card to the correct element, look for an element on the periodic table so that if you add the number of neutrons on your card to the protons of the element, you will get close to the atomic mass for that element. For example, you may have a card that says that the atom you are looking for has 5 neutrons. Lesson 3 You would look at the periodic table to find an atom that you could add 5 to its number of protons that would give you a sum close to the atomic mass given for that element. The answer is beryllium (Be), which has 4 protons and an atomic mass of 9.01. Note: There are a few neutron cards that have two possible correct elements instead of just one: • 6 Neutrons—Boron or Carbon • 10 Neutrons—Fluorine or Neon • 12 Neutrons—Sodium or Magnesium • 16 Neutrons—Phosphorous or Sulfur • 20 Neutrons—Potassium or Calcium EXPLORE 4. Have groups work together to place each card with its correct atom. 5. Discuss the placement of the cards for two or three atoms. Select two or three atoms and review whether the cards were placed correctly. This review will help reinforce the concepts about the structure of atoms and help students determine the number of protons, electrons, and neutrons in each type of atom. Have students begin filling out the activity sheet with the following information: • Number of protons • Number of electrons • Number of neutrons (usually) EXTEND 6. Introduce students to their element project and an online resource that they can use. Assign each student to an element. Include the first 20 elements and any other elements that you find interesting so that each student can research and present their own. Each student should find and present some basic information about their element to the class. The presentation can be in the form of a poster, pamphlet, PowerPoint presentation Lesson 3 or other form. The presentations should be short and can include: atom name, atomic number, derivation of name, when and where discovered, natural sources of the element, major uses, and any other information you find important. Lesson 3 QUICK LAB Recognizing Patterns In this lab, you will construct a table that organizes elements according to atomic number. You will then use the table to predict the atomic numbers and identities of other elements. PROCEDURE Fill out the empty cells in the table below using the elements given. Place elements in the table from left to right in order of increasing atomic number. Be sure to label each element with its correct symbol, atomic number, and mass. OBJECTIVE • Predict the atomic numbers of elements in a table. MATERIAL For each group • periodic table of the elements Elements: Phosphorus (P, 15, 30.9) Arsenic (As, 33, 74.9) Sulfur (S, 16, 32.0) Bromine (Br, 35, 79.9) Chlorine (Cl, 17, 35.5) Using the data from the first two rows, predict the atomic numbers of the elements in the shaded cells. Write the numbers in the appropriate shaded cells. Now look at a periodic table of elements. Complete the table with the names, symbols, and atomic masses of the elements in the shaded cells. Lesson 3 QUICK LAB Predicting Properties In this lab, you will create a graph of atomic radius values and use the graph to interpret trends in the periodic table. You will recognize that elements are grouped in the periodic table according to similarities of their properties. PROCEDURE On your graph paper, create a line graph using the values in the data table below. Label the x-axis “Atomic Number” and the yaxis “Atomic Radius.” Review the range of values in the table to determine an appropriate scale for your graph. Atomic Number Atomic Radius (picometers) Atomic Number Atomic Radius (picometers) 1 80 19 280 2 50 20 220 3 210 21 210 4 140 22 200 5 120 23 190 6 90 24 190 7 80 25 180 8 70 26 170 9 60 27 170 10 ? 28 160 11 220 29 160 12 180 30 150 13 170 31 ? 14 150 32 150 15 130 33 130 16 110 34 120 17 100 35 110 18 ? 36 100 OBJECTIVES • Graph values for atomic radii. • Interpret trends in the periodic table of elements. MATERIALS For each student • graph paper • periodic table Lesson 3 Quick Lab continued Describe the pattern you see in the graph. _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ Compare your graph to the periodic table of elements. How does the pattern relate to the structure in the periodic table? _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ What trends do you observe about the the relationship between atomic radius and atomic number in the periodic table? _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ Based on these trends, what are the approximate values of the atomic radii of elements 10, 18, and 20? _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ Lesson 3 Lesson 3 NOVA VIDEO 1. What is the name of the movie we are watching? _____________________________________________________ 2. What is the first element they are digging out from the ground? What is the symbol? _____________________________________________________ _____________________________________________________ 3. What is one unique thing about gold? ______________________________________________________ ______________________________________________________ 4. What will you be able to see after the gold assay is complete? _____________________________________________________ _____________________________________________________ 5. Why is Gold stand-offish? _____________________________________________________ _____________________________________________________ _____________________________________________________ _____________________________________________________ 6. Why does David have to wear the special silver suit? _____________________________________________________ _____________________________________________________ _____________________________________________________ 7. How much money is one gold bar worth? _____________________________________________________ _____________________________________________________ Lesson 3 8. What is the next element in the movie? What is the atomic number? _____________________________________________________ _____________________________________________________ 9. How much copper is sold a year? _____________________________________________________ _____________________________________________________ 10. What do you get when you combine copper and tin? _____________________________________________________ _____________________________________________________ 11. Why do bellmakers still make bells out of bronze? _____________________________________________________ _____________________________________________________ 12. What happens in if there is too much tin mixed with copper in a bell? _____________________________________________________ _____________________________________________________ 13. How far do you have to zoom in to see the atoms on the electron microscope? _____________________________________________________ _____________________________________________________ 14. How big is the machine needed to see atoms? _____________________________________________________ _____________________________________________________ Lesson 3 15. Why can you only get a little information from the atom microscope? _____________________________________________________ _____________________________________________________ 16. What is the symbol based on? _____________________________________________________ _____________________________________________________ 17. Why are noble gasses noble? _____________________________________________________ _____________________________________________________ _____________________________________________________ 18. What was chlorine used for in World War I? _____________________________________________________ _____________________________________________________ _____________________________________________________ 19. What reaction are they doing with sodium? _____________________________________________________ _____________________________________________________ 20. What happens in the reaction? _____________________________________________________ _____________________________________________________ _____________________________________________________ _____________________________________________________ Lesson 3 NOVA VIDEO 1. What is the name of the movie we are watching? _____________________________________________________ 2. How did David and the scientist collect evidence from the explosion site? _____________________________________________________ _____________________________________________________ _____________________________________________________ 3. What is the difference in a combustion reaction? _____________________________________________________ _____________________________________________________ 4. How do you control the speed of combustion? _____________________________________________________ _____________________________________________________ 5. What are the elements of life? _____________________________________________________ _____________________________________________________ _____________________________________________________ _____________________________________________________ 6. What did they buy to represent CHNOPS? _____________________________________________________ _____________________________________________________ _____________________________________________________ 7. Why is carbon the backbone of all living things? _____________________________________________________ _____________________________________________________ Lesson 3 8. How does a tire relate to a human? _____________________________________________________ _____________________________________________________ 9. Where did the microbiologist take David? _____________________________________________________ _____________________________________________________ 10. What type of life form evolved when the Earth cooled? _____________________________________________________ _____________________________________________________ 11. What did the Earth do as the cyanobacteria produced oxygen? _____________________________________________________ _____________________________________________________ 12. What is the process that turns hydrogen into helium? _____________________________________________________ _____________________________________________________ 13. Why do we want to reproduce the process of solar fusion on Earth? _____________________________________________________ _____________________________________________________ 14. What is the temperature of the energy that is produced during fusion? _____________________________________________________ _____________________________________________________ Lesson 3 15. What does sand look like under a microscope? _____________________________________________________ _____________________________________________________ 16. Why do scientists add different things to glass? _____________________________________________________ _____________________________________________________ 17. What products do you think we use that have glasses that is almost unbreakable? _____________________________________________________ _____________________________________________________ _____________________________________________________ 18. What products are rare earth’s a part of? _____________________________________________________ _____________________________________________________ _____________________________________________________ 19. What happens to cause an electric current going from rare earth metals to shark fins? _____________________________________________________ _____________________________________________________ 20. What is carbon dating? _____________________________________________________ _____________________________________________________ _____________________________________________________ _____________________________________________________ Lesson 3 21. What did you find most interesting from this video? Why did this topic interest you? _____________________________________________________ _____________________________________________________ _____________________________________________________ _____________________________________________________ 22. If you could research one thing about elements and reactions what would it be? Why? _____________________________________________________ _____________________________________________________ _____________________________________________________ _____________________________________________________ Lesson 4 Standard: MS1 Matter and its Interactions PE: MS-PS15. Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved. Practice: Disciplinary Core Idea: Crosscutting Concept: Developing and Using Models PS1.B: Chemical Reactions Energy and Matter Obtaining, Evaluating, and Communicating Information Science Models, Laws, Mechanisms, and Theories Explain Natural Phenomena Objectives Students will be able to identify the difference between Ionic and Covalent Bonds Students will be able to draw and model a Bohrs Model based on the location of the element in the periodic table. Students will be able to define the properties of Ionic, Covalent and Metallic Bonds. Classroom Activities Assessments Review and basics of bonding. Bonding Quiz Build a Bohr’s Model. Ionic, Covalent, and Metallic Online Virtual Lab www.thinkcentral.com Chemical Bonding Worksheet-Determining Bonds based on Metal and Non-metal Combination Modeling Bonding class rotation activity Ionic Bonding Worksheet Bohr Model Compounds Differentiation-Students continue to get harder worksheets as they complete easier ones. They are grouped at tables based on the worksheet they are on. Lesson 4 Atomic Bonding A general guide Lesson 4 Review! Periodic Table contains all the elements Atomic Number Atomic Symbol Atomic Mass Element Name All elements are made of protons, neutrons, and electrons Lesson 4 Neutrons Neutral charge Make up part of the atomic mass with protons Not always the same number as protons and electrons Atomic mass-Number of protons=number of neutrons Lesson 4 Protons and Electrons Atomic Number=Number of Protons Protons have a positive Charge Electrons have a negative charge Number of electrons SHOULD equal the number of protons Lesson 4 Electron Energy levels Energy levels correspond to the periods on periodic table 2 Electrons can be in the first energy level 8 electrons can be in the second and third 18 electrons can be in the fourth - - - - - - - - - Lesson 4 Valence Electrons Electrons in the farthest energy level Used in both types of bonding Can be gained or lost or combined with another element Cause the atom to be more stable or lest stable Lesson 4 Covalent Bonding Bond formed with two elements share electrons Force is the attractions of shared electrons to each nucleus Each bond forms a molecule Mostly form between non-metals Can be bonded into double and triple bonds Lesson 4 Ionic Bonding Attraction between two oppositely charged ions Ions are formed by atoms gaining and losing electrons Goal is to make the atom more stable Bond forms because of the attraction between positive and negative ions Form Compounds Lesson 4 Electron Dot Diagrams Shows the number of valence electrons Aids in showing how atoms can bond To bond, atoms either add up to 8 or lose electrons Lesson 4 Valence Electrons Electrons in the farthest energy level Used in both types of bonding Can be gained or lost or combined with another element Cause the atom to be more stable or lest stable Lesson 4 Charges In the periodic Table Noble Gases are special Have + or – ion based on how the element can become more stable Every element in the same group with have the same ion Let’s practice a little Lesson 4 Homework Pg 27 Writing in Science Pretend you are the size of an atom, observing a reaction between potassium and fluorine. Write an account of the formation of an ionic bond as the atoms react. Tell what happens to the valence electrons on each atom and how each atom I changed by losing or gaining electrons. Lesson 4 S.T.E.M. LAB Build a Bohr Model In this lab, you will work in small groups to create a Bohr model of a selected atom. You will have many different materials available, but your group must decide how to assemble the materials to create your model. When creating your model, your group should try to use all the materials provided. Remember that atoms of elements in the first period of the periodic table have one energy level that can contain a maximum of two electrons. In the second period, atoms have two energy levels. The second energy level can contain a maximum of eight electrons. Atoms of elements in the third period have three energy levels. For elements in the third period, the third energy level can also hold a maximum of eight electrons. Your group will determine where all the electrons in your model belong and will make predictions about how your atom could bond with other atoms. PROCEDURE ASK A QUESTION How will building a Bohr model help you understand the bonding of atoms? ____________________________________________________ ____________________________________________________ BUILD A MODEL Select an element from the first three periods of the periodic table. Write your choice below. ____________________________________________________ OBJECTIVES • Create a Bohr model of an atom. • Distinguish the valence electrons. • Analyze how valence electrons affect how atoms bond with other atoms. MATERIALS For each group • aluminum foil • ball, foam • cardboard • clay, modeling • glue, white • gumdrops • hole punch • marker • marshmallows, miniature • paper brad • paper, construction • paper, tissue • periodic table, copy • pipe cleaners • plate, paper • scissors • string For each student • safety goggles Lesson 4 S.T.E.M. Lab continued Work with your group to build a Bohr model of your atom. Remember that Bohr models show the nucleus in the center of the model, and each energy level is represented as a ring around the nucleus, as shown in the image below. MAKE OBSERVATIONS How many valence electrons are in your model? _________________________________________________________________________ FORM A HYPOTHESIS Given the number of valence electrons, how will atoms of your element react with other atoms? Write a hypothesis below and be sure it includes a “because” statement. _________________________________________________________________________ _________________________________________________________________________ Study the periodic table and determine at least two other elements whose atoms could form a bond with your atom. Write the elements below. _________________________________________________________________________ What would you need to test your hypothesis? _________________________________________________________________________ DRAW CONCLUSIONS Analyzing Models How accurately did your model represent your chosen element? _________________________________________________________________________ Lesson 4 S.T.E.M. Lab continued Explaining Hypotheses Explain why you believe your atom would bond with the atoms you identified in Step 6. Be sure to include what will happen to the valence electrons. If you believe your atom would not form bonds, explain why. _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ Applying Conclusions How does the common compound salt (sodium chloride) illustrate the ideas in this lab? _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ Describing Constraints In what ways do Bohr models fail to accurately represent atoms? _________________________________________________________________________ _________________________________________________________________________ TO THE ESSENTIAL QUESTION Applying Concepts What did you find to be the determining characteristic of an atom that defines how it bonds with another atom? _________________________________________________________________________ _________________________________________________________________________ Lesson 4 S.T.E.M. LAB Build a Bohr Model In this lab, you will work in small groups to create a Bohr model of a selected atom. You will have many different materials available, but your group must decide how to assemble the materials to create your model. Your group may choose not to use all the materials that are available; you will be asked to provide justification for choosing the materials you use in your models. Your group will determine where all the electrons in your model belong, and you will make predictions about how your atom could bond with other atoms. PROCEDURE ASK A QUESTION How will building a Bohr model help you understand the bonding of atoms? ____________________________________________________ ____________________________________________________ BUILD A MODEL Select an element from the first three periods of the periodic table. Write your choice below. ____________________________________________________ Work with your group to build a Bohr model of an atom of the element you chose. Remember that Bohr models show the nucleus in the center of the model, and each energy level is represented as a ring around the nucleus. MAKE OBSERVATIONS How many valence electrons are in your model? ____________________________________________________ OBJECTIVES • Create a Bohr model of an atom. • Distinguish the valence electrons. • Analyze how valence electrons affect how atoms bond with each other. MATERIALS For each group • aluminum foil • ball, foam • cardboard • clay, modeling • glue, white • gumdrops • hole punch • marker • marshmallows, large • marshmallows, miniature • paper brad • paper, construction • paper, tissue • periodic table, copy • pipe cleaners • plate, paper • scissors • string For each student • safety goggles Lesson 4 S.T.E.M. Lab continued FORM A HYPOTHESIS Given the number of valence electrons, how will the atoms of your element react with other elements? Write a hypothesis below and be sure it includes a “because” statement. _________________________________________________________________________ _________________________________________________________________________ Study the periodic table and determine at least two other elements with which your element could form a bond. Write the elements below. _________________________________________________________________________ What would you need to test your hypothesis? _________________________________________________________________________ DRAW CONCLUSIONS Analyzing Models How accurately did your model represent your chosen element? _________________________________________________________________________ Defending Methods Explain why you selected the materials you used and why you chose not to use other materials. _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ Explaining Hypotheses Explain why you believe your atom would bond with the atoms you identified in Step 6. Be sure to include what will happen to the valence electrons. If you believe your element is stable, explain why. _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ Applying Conclusions How does the common compound salt (sodium chloride) illustrate the ideas in this lab? _________________________________________________________________________ _________________________________________________________________________ Lesson 4 S.T.E.M. Lab continued Describing Constraints In what ways are Bohr models not fully accurate representations of atoms? _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ TO THE ESSENTIAL QUESTION Applying Concepts What did you find to be the determining characteristic of an atom that defines how it bonds with another atom? _________________________________________________________________________ _________________________________________________________________________ Lesson 4 Creating a Bohr’s Model Name: ________________________ 1 No electrons are present. 2 Number of shells and electrons is incorrect. Nucleus Representation Nucleus is missing particles. Improper number of neutrons or protons are present in the nucleus but particles are not mixed up. Design Design does not reflect any models of an atom. Construction Poorly constructed model with no design of atomic models. Model does not represent one of the atoms from the periodic table. Electron Shell Representation Accuracy of Whole Model Total /25 3 Proper number of electrons are represented but the number of shells is incorrect. Improper number of neutrons or protons are present in the nucleus but particles are mixed up. Design is not Design is not based on Bohr’s based on model of an Bohr’s model atom, but does of an atom, reflect a different but is correct model of an atom in the model with mistakes. of an atom it represents. Poorly Poorly constructed constructed model with no model with design in mind. design in mind. Model represents one of the atoms from the periodic table, but is not identifiable because of misconstruction. Model represents one of the atoms from the periodic table but is not identifiable. 4 Proper number of shells are represented but not the proper number of electrons in each shell. Proper number of neutrons and protons are in nucleus but not mixed up. 5 Proper number of shells are represented with the proper number of electrons in each shell Proper number of neutrons and protons are mixed in the nucleus. Design is based on Bohr’s model of an atom but includes mistakes. Design is based on Bohr’s model of an atom. Well constructed model with design not in mind. Well constructed model, with design in mind Model represents one of the atoms from the preriodic table that is identifiable with some manipulation of the model. Model represents one of the atoms from the periodic table that is identifiable. Lesson 4 Lesson 4 Lesson 4 Lesson 4 Lesson 4 Lesson 4 Lesson 4 Chemical Bonding Worksheet Ionic Bond Covalent Bond Metallic Bond between a Metal and Non-Metal between a Non-Metal and Non-Metal between a Metal and Metal (M + NM) (NM + NM) (M+ M) Determine if the elements in the following compounds are metals or non-metals. Describe the type of bonding that occurs in the compound. Compound NO2 NaCl SO2 PO43MgBr2 CaO H2O K2 O O2 CuCl2 NO2TiO2 HF Rb2S Fe2O3 Element 1 (metal or nonmetal?) N = non-metal Element 2 (metal or nonmetal?) O = non-metal Bond Type covalent Lesson 4 QUICK LAB Modeling Bonding Chemical bonds hold atoms together. Ions form ionic bonds. Positive ions can only bond with negative ions, and negative ions can only bond with positive ions. PROCEDURE Wear the name tag your teacher gives you. What is the atom on your name tag? Does it have a positive or negative ion? ____________________________________________________ ____________________________________________________ ____________________________________________________ With your class, form two circles, one inside the other. Walk around the circle. If you are in the outside circle, walk clockwise. If you are in the inside circle, walk counter clockwise. When your teacher says “stop,” look at the name tag of the student opposite you. OBJECTIVE • Model the formation of an ionic bond. MATERIALS For each student • atom name tag • Bonding Time worksheet • pencil • reference materials Ask yourself if the atom on your name tag can bond with the atom of the student across from you. If not, do nothing. If yes, fill out the information in the Bonding Time worksheet. Write the compound and make up a name for it. Research one of the bonds in your worksheet to see what happens when this bond forms. Share your findings with the class. _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ _________________________________________________________________________ Lesson 4 Quick Lab continued Bonding Time Positive Ion Negative Ion Compound Compound Name Lesson 4 Lesson 4 Lesson 4 Name:_________________________ Date: _________________________ Period: _______________________ Bohr’s Model Compounds Directions: Draw the Bohr’s model of the following reactions. Be sure to include the reactants as well as the products. Label the formulas covalent or ionic. If the formula contains a metalloid consider it a covalent bond. Li + F => LiF Be + S => BeS Mg + o => MgO Ca + S => CaS Na + F => NaF Al + N => AlN C + Si => CSi B + N => BN Li + Cl => LiCl Be + O => BeO Lesson 4 B + P => BP K + F => KF H + Cl => HCl Lesson 4 Name: ________________________ Date: _________________________ Period: _______________________ Bohr’s Model Compounds II Directions: Draw the Bohr’s model of the following reactions. Be sure to include the reactants as well as the products. Label the formulas covalent or ionic. If the formula contains a metalloid consider it a covalent bond. 2H + O => H2O Cl + Cl =>Cl2 S + 2O => SO2 2Na + O => Na2O N + 2O => NO2 2K + O => K2O Ca + 2Cl => CaCl2 Ca + 2H => CaH2 F + F => F2 2K + O => K2O 2 Be + C => Be2C Mg + 2F => MgF2 Lesson 4 2Mg + Si => Mg2Si 2Ca+ Si => Ca2Si Be + 2F => BeF2 Lesson 4 Name: ________________________ Date: _________________________ Period: _______________________ Bohr’s Model Compounds III Directions: Draw the Bohr’s model of the following reactions. Be sure to include the reactants as well as the products. Label the formulas covalent or ionic. If the formula contains a metalloid consider it a covalent bond. N + 3H=> NH3 S + 3O => SO3 C + 4Cl => CCl4 3Na + N => Na3N 3O => O3 P + 3Cl => PCl3 Li + O + H => LiOH 3N => N3 Na + C + N => NaCN Na + Cl + O => NaClO 2Na + 2O => Na2O2 Al + 3Cl =>AlCl3 Lesson 4 2H + 2O => H2O2 N + 4H => NH4 Ca + C + 3O => CaCO3 Lesson 4 Name: ________________________ Date: ________________________ Period: _______________________ BONDING QUIZ Directions: Using your notes answer the following questions with as much detail as possible. 1. What are the properties of an Ionic Bond. ______________________________________ ______________________________________ ______________________________________ ______________________________________ ______________________________________ 2. What are the properties of a Covalent Bond. ______________________________________ ______________________________________ ______________________________________ ______________________________________ 3. What are the properties of a Metallic Bond. ______________________________________ ______________________________________ ______________________________________ ______________________________________ 4. Draw the Bohr’s models of the formula Na + Cl => NaCl. Label if this is a Covalent or Ionic Bond. Explain how you know. _____________________________________________ _____________________________________________ Lesson 4 5. Draw the Bohr’s models of the formula H + F => HF. Label if this is a Covalent or Ionic Bond. Explain how you know? _____________________________________________ _____________________________________________ 6. What type of bond is the following compound? How do you know? _____________________________________________ _____________________________________________ 7. What type of bond is the following compound? How do you know? +2 -2 _____________________________________________ _____________________________________________ Lesson 4 Name: ________________________ Date: ________________________ Period: _______________________ BONDING QUIZ-Modified Directions: Using your notes answer the following questions with as much detail as possible. 1. What are the properties of an Ionic Bond. __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ 2. What are the properties of a Covalent Bond. _____________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Lesson 4 3. What are the properties of a Metallic Bond. ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ 4. Draw the Bohr’s models of the formula Na + Cl => NaCl. Label if this is a Covalent or Ionic Bond. Explain how you know. __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ Lesson 4 5. Draw the Bohr’s models of the formula H + F => HF. Label if this is a Covalent or Ionic Bond. Explain how you know? __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ Lesson 4 6. What type of bond is the following compound? How do you know? __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ Lesson 4 7. What type of bond is the following compound? How do you know? +2 -2 __________________________________________________________ ________________________________________________________ _____________________________________________ _____________________________________________ _____________________________________________ _________________________________________ Lesson 5 Standard: MS-PS1 Matter and its Interactions PE: MS-PS15. Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved Practice: Disciplinary Core Idea: Crosscutting Concept: Developing and Using Models PS1.B: Chemical Reactions Energy and Matter Objectives Classroom Activities Assessments Mini Lab-Drawing Electron Dot Diagrams/Lewis Structures Students model elements using Electron Dot Diagrams/Lewis Structure Students will be able to draw chemical reactions using Lewis Structures/Electron Dot Diagrams Students will be able to identify a covalent or ionic bond based on the Lewis structure. Content Mastery-Why do Atoms Combine worksheet Rules For Drawing Lewis Structures Differentiation Activity-Each student gets progressively more challenging worksheets. Students are grouped at tables based on the worksheet they are working on. Ionic Bonding Comic Basics of Bonding-Ionic Bonds-drawing the entire ionic reaction of two elements Bonding Basics-Covalentdrawing the entire covalent reactions between two elements Electron Dot Diagram Quiz Lesson 5 Lesson 5 Lesson 5 Rules for drawing Lewis structures: 1. Determine the total number of valence electrons (outer shell electrons) for the compound. This corresponds to the "A" group the atom belongs on the periodic table, not the atomic number. For example, CO2: carbon is in group IVA and has 4 valence electrons, oxygen is in group VIA and each has 6 valence electrons, giving a total of 16 valence electrons for the molecule. 2. Determine the central atom (often the atom that is least electronegative). a. carbon or silicon are always in the middle. b. hydrogen is never in the middle. c. oxygen is usually not in the middle d. the halogens (F, Cl, Br, I) are usually not in the middle. 3. Draw the basic structure, the central atom surrounded by the remaining atoms. 4. Between the central atom and each surrounding atom, place two electrons or a line to designate a single covalent bond (i.e. C-H) composed of a shared electron pair. 5. The octet rule: Surround each atom with eight electron dots (four pairs of electrons). *Remember, the covalent bond counts as two electrons for each atom to which it is attached. *Remember, hydrogen can only have two valence electrons. 6. Count the number of electrons; each line (covalent bond) counts as two, each dot as one. a. If the number of electrons equals the valence electrons in step 1, this is the Lewis structure. b. If you have more electrons than in step one, you must replace one or more single bonds with multiple bonds. When you draw a double bond, you must erase two electrons from each atom attached to the double bond (erase four electrons total) to satisfy the octet rule. *Hydrogen cannot make a double bond. Halogens do not make double bonds Lesson 5 PART A: Molecule CO2 SO3 SO2 CH4 NH3 H2O Val e-` Lewis Structure Polar? Lesson 5 Part B: More Lewis Structures with one central atom Molecule CF2Cl2 HCN PBr3 CH2O N2O Val e-` Lewis Structure Polar? Lesson 5 PART C: Negative charged molecules: add one valence electron for every negative charge. Molecule NO3- CO32- SO32- Val e-` Lewis Structure Polar? Lesson 5 PART D: More than one central atom. Molecule C2H6 C2H4 C2H2 CH3OH C2H4Cl2 Val e-` Lewis Structure Polar? Lesson 5 Postlab Question: In some chemistry courses, the instructor requires students to purchase a model kit. Suppose you were in such a course and decided to economize by making your kit out of different colored gumdrops and toothpicks. What problems might you have in using your kit? ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ Lesson 5 Lesson 5 Lesson 5 Lesson 5 Lesson 5 Lesson 5 Lesson 5 Lesson 5 Lesson 5 Lesson 5 Electron Dot Diagram Quick Quiz Draw the Electron Dot Diagram of the following Compound : HCN Draw the Electron Dot Diagram of the following Compound : SO2 Lesson 5 Electron Dot Diagram Quick Quiz-Modified Draw the Electron Dot Diagram of the following Compound : HCN Draw the Electron Dot Diagram of the following Compound : SO2 Lesson 6 Standard: MS-PS-1 PE: MS-PS11. Develop models to describe the atomic composition of simple molecules and extended structures MS-PS13. Gather and make sense of information to describe that synthetic materials come from natural resources and impact society. MS-PS15. Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved. Practice: Disciplinary Core Idea: Objectives Classroom Activities Crosscutting Concept: Assessments Study Guide Jeopardy Game Students will learn how to prepare for a written exam. Intro to Chemistry Test (Modified Test Included) Lesson 6 Name: ___________________ Date:____________________ Class Period:_______________ Intro to Chemistry Study Guide 1. Atoms are made of three particles. What are the names of the three particles? 2. Which two particles are in the nucleus? 3. What is the atomic theory? 4. We use the Bohr’s model to represent atoms. Why is Bohr’s model a misrepresentation of an atom? 5. The modern atomic model contains two key ideas. They are _______. 6. What are valence electrons? 7. What is a period? What is a group? 8. Why is group 18 special? 9. What do we call an atom that has a positive or negative charge? 10. What did each model look like in the atomic theory and who discovered the model? 11. What makes ionic and covalent bonds different? Lesson 6 12. How did the atomic theory continue to change as new scientists investigated atoms? 13. Label parts of an element from the periodic table on the line 6 C 12.011 14. Draw two Bohr’s Models in the boxes 15. Draw electron dot diagrams in the boxes Lesson 6 16. Draw the electron dot diagram of a covalent bond in one box and an ionic bond in the other 17. Review the parts of a graph. 18. How does that atomic radaii change across groups? How does the atomic radaii change across periods? Lesson 6 Name: ___________________ Date:____________________ Class Period:_______________ Intro to Chemistry Test Directions: Circle the best answer for each multiple choice question. (1 point each) 19. Atoms are made of three particles. What are the names of the three particles? a. Neutrons, quarks, chromosomes b. Protons, chromosomes, electrons c. Neutrons, protons, electrons d. Electrons, protons, quarks 20.Which two particles are in the nucleus? a. Neutrons and protons b. Neutrons and chromosomes c. Protons and electrons d. Electrons and quarks 21.What is the atomic theory? a. The way atoms bond together b. A series of models that developed from experimental evidence c. A series of calculations from the periodic table d. Observations based on interactions of elements 22.Thomson believed the atom looked like a _______________. a. magic 8 ball b. blueberry muffin c. solar system d. cloud 23.Rutherford believed atoms had a positively charged nucleus. What experiment did he do to figure this out? a. Built a model out of marshmallows Lesson 6 b. Graphed atomic radaii c. Shot a particle beam into gold foil d. Used a baseball diamond to map out how far an electron is away from the nucleus 24.We use the Bohr’s model to represent atoms. Why is Bohr’s model a misrepresentation of an atom? a. Electrons don’t float in space and there are no neutrons in the nucleus b. Electrons are found in the nucleus and protons float in space c. There are no neutrons in the nucleus and protons orbit the atom d. Electrons do not orbit the atom and neutrons are found in the nucleus 25.The modern atomic model contains two key ideas. They are _______. a. Neutrons are found in the nucleus, electrons are found in a cloud b. Neutrons are found in a cloud, electrons are found in the nucleus c. Protons orbit the atom, electrons have no charge d. Electrons orbit the atom, no neutrons are in the nucleus. 26.What are valence electrons? a. All the electrons in an atom b. Electrons that are held loosely and have the highest energy level c. Electrons in the nucleus d. Electrons that have a low energy level in the cloud 27.What is a period? a. The columns on the periodic table that have atoms with the same properties b. The columns on the periodic table that have atoms with decreasing radaii c. The rows on the periodic table that have decreasing number of protons d. The rows on the periodic table that have atoms with increasing atomic number Lesson 6 28.What is a group? a. The columns on the periodic table that have atoms with the same properties b. The columns on the periodic table that have atoms with decreasing radaii c. The rows on the periodic table that have decreasing number of protons d. The rows on the periodic table that have atoms with increasing atomic number 29.Why is group 18 special? a. They are all metals b. They have all their valence electrons filled up and are stable c. They bond very easily with everything d. They are metalloids 30.How many valence electrons does group 2 have? a. 2 b. 4 c. 6 d. 8 31.How many valence electrons does group 16 have? a. 2 b. 4 c. 6 d. 8 32.What is the unit we use for atomic radius? a. Millimeters b. Centimeters c. Kilometers d. Picometers 33.What do we call an atom that has a positive or negative charge? Lesson 6 a. b. c. d. Bond Ion Chromosome Base Pair Directions: Determine if each compound is a covalent bond or ionic bond 34. Sodium Bromide (NaBr) ____________________ 35.Lithium oxide (Li2O) 36.Water (H2O) 37.Table salt (NaCl) 38.Methane (CH4) ____________________ ____________________ ____________________ ____________________ Directions: Label each atomic theory based on who discovered the model (1 point each) ++ ++ 21.________________ + + 22.________________ 0 23._________________ 24._________________ Lesson 6 25.How did the atomic theory continue to change as new scientists investigated atoms? Please answer in complete sentences. (2 points) __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ Directions: Label parts of an element from the periodic table on the line (1 point each) 6 27. C 12.011 26. 28. Lesson 6 Directions: Choose three atoms from the periodic table and draw a Bohr’s Model of each atom in the box (2 points each) 29. 30. 31. 32. Why do we use the Bohr’s model of atoms if the theory is no longer accepted? Please answer in complete sentences. (2 points) _____________________________________________________________ _____________________________________________________________ _____________________________________________________________ _____________________________________________________________ Lesson 6 Directions: Draw electron dot diagram for each of the following elements (1 point each) 32. Li 33. P 34. Ca Directions: Draw electron dot diagrams for each of the following compounds. Label as ionic or covalent (2 points each) 35.O2 37. NaCl Lesson 6 Directions: Using the following data table, graph the atomic radaii. Remember to include all parts of a graph! (6 points) Atomic Number 4 12 20 38 56 Element Be Mg Ca Sr Ba Radius (in picometers) 112 160 197 215 222 Lesson 6 38. Do the points curve or stay in a straight line? Please answer in complete sentences. (1 point) _____________________________________________________________ _____________________________________________________________ _____________________________________________________________ _____________________________________________________________ 39. What does the trend show in relation to the size of atoms? Please answer in complete sentences. (2 points) _____________________________________________________________ _____________________________________________________________ _____________________________________________________________ _____________________________________________________________ _____________________________________________________________ _____________________________________________________________ 40. How does this relate to other groups on the periodic table? Please answer in complete sentences. (2 points) _____________________________________________________________ _____________________________________________________________ _____________________________________________________________ _____________________________________________________________ _____________________________________________________________ _____________________________________________________________ Lesson 6 Name: ___________________ Date:____________________ Class Period:_______________ Intro to Chemistry Test-Modified Directions: Circle the best answer for each multiple choice question (1 point each) 1. Atoms are made of three particles. What are the names of the three particles? a. Neutrons, quarks, chromosomes b. Protons, chromosomes, electrons c. Neutrons, protons, electrons 2. Which two particles are in the nucleus? a. Neutrons and protons b. Protons and electrons c. Electrons and quarks 3. What is the atomic theory? a. The way atoms bond together b. A series of models that developed from experimental evidence c. A series of calculations from the periodic table 4. Thomson believed the atom looked like a _______________. a. magic 8 ball b. blueberry muffin Lesson 6 c. solar system 5. Rutherford believed atoms had a positively charged nucleus. What experiment did he do to figure this out? a. Built a model out of marshmallows b. Graphed atomic radaii c. Shot a particle beam into gold foil 6. We use the Bohr’s model to represent atoms. Why is Bohr’s model not a good representation of an atom? a. Electrons don’t float in space and there are no neutrons in the nucleus b. There are no neutrons in the nucleus and protons orbit the atom c. Electrons do not orbit the atom and neutrons are found in the nucleus 7. The modern atomic model contains two key ideas. They are _______. a. Neutrons are found in the nucleus, electrons are found in a cloud b. Neutrons are found in a cloud, electrons are found in the nucleus c. Protons orbit the atom, electrons have no charge Lesson 6 8. What are valence electrons? a. All the electrons in an atom b. Electrons that are held loosely and have the highest energy level c. Electrons that have a low energy level in the cloud 9. What is a period? a. The columns on the periodic table that have atoms with the same properties b. The columns on the periodic table that have atoms with decreasing radaii c. The rows on the periodic table that have atoms with increasing atomic number 10. What is a group? a. The columns on the periodic table that have atoms with the same properties b. The columns on the periodic table that have atoms with decreasing radaii c. The rows on the periodic table that have atoms with increasing atomic number 11. Why is group 18 special? a. They are all metals b. They have all their valence electrons filled up and are stable Lesson 6 c. They bond very easily with everything 12. How many valence electrons does group 2 have? a. 2 b. 6 c. 8 13. How many valence electrons does group 16 have? a. 2 b. 6 c. 8 14. What is the unit we use for atomic radius? a. Centimeters b. Kilometers c. Picometers 15. What do we call an atom that has a positive or negative charge? a. Bond b. Ion c. Base Pair Lesson 6 Directions: Determine if each compound is a covalent bond or ionic bond (1 point each) 16. Sodium Bromide (NaBr) ____________________ 17. Lithium oxide (Li2O) ____________________ 18. Water (H2O) 19. Table salt (NaCl) ____________________ 20. Methane (CH4) ____________________ ____________________ Directions: Label each atomic theory based on who discovered the model (1 point each) 0 21.________________ 22._________________ 23. How did the atomic theory continue to change as new scientists investigated atoms? Please answer in complete sentences. (2 points) ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ __________________________________________________________________________ Lesson 6 Directions: Label parts of an element from the periodic table on the line (1 point each) 24. 6 C 25. 26. 12.011 Directions: Choose three atoms from the periodic table and draw a Bohr’s Model of each atom in the box (2 points each) 27. 28. Lesson 6 29. Why do we use the Bohr’s model of atoms if the theory is no longer accepted? Please answer in complete sentences. (2 points) ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ Directions: Draw electron dot diagram for each of the following elements (1 point each) 30. Li 31. P Directions: Draw electron dot diagrams for each of the following compounds. Label as ionic or covalent (2 points) 32. O2 Lesson 6 Directions: Using the following data table, graph the atomic radaii. Remember to include your axis labels and a title! (6 points) Atomic Number 4 12 20 38 56 Element Be Mg Ca Sr Ba Radius (in picometers) 112 160 197 215 222 Lesson 6 33. Do the points curve or stay in a straight line? Please use complete sentences. (1 point) ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ 34. What does the trend show in relation to the size of atoms? Please use complete sentences. (2 points) ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ 35. How does this relate to other groups on the periodic table? Please use complete sentences. (2 points) ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ ______________________________________________________ Lesson 6 Name: ___________________ Date:____________________ Class Period:_______________ Intro to Chemistry Test-Answer Key Directions: Circle the best answer for each multiple choice question (1 point each) 39. Atoms are made of three particles. What are the names of the three particles? a. Neutrons, quarks, chromosomes b. Protons, chromosomes, electrons c. Neutrons, protons, electrons d. Electrons, protons, quarks 40. Which two particles are in the nucleus? a. Neutrons and protons b. Neutrons and chromosomes c. Protons and electrons d. Electrons and quarks 41. What is the atomic theory? a. The way atoms bond together b. A series of models that developed from experimental evidence c. A series of calculations from the periodic table d. Observations based on interactions of elements 42. Thomson believed the atom looked like a _______________. a. An magic 8 ball b. A blueberry muffin c. A solar system d. A cloud 43. Rutherford believed atoms had a positively charged nucleus. What experiment did he do to figure this out? a. Built a model out of marshmallows b. Graphed atomic radaii c. Shot a particle beam into gold foil d. used a baseball diamond to map out how far an electron is away from the nucleus Lesson 6 44. We use the Bohr’s model to represent atoms. Why is Bohr’s model not a good representation of an atom? a. Electrons don’t float in space and there are no neutrons in the nucleus b. Electrons are found in the nucleus and protons float in space c. There are no neutrons in the nucleus and protons orbit the atom d. Electrons do not orbit the atom and neutrons are found in the nucleus 45. The modern atomic model contains two key ideas. They are _______. a. Neutrons are found in the nucleus, electrons are found in a cloud b. Neutrons are found in a cloud, electrons are found in the nucleus c. Protons orbit the atom, electrons have no charge d. Electrons orbit the atom, no neutrons are in the nucleus. 46. What are valence electrons? a. All the electrons in an atom b. Electrons that are held loosely and have the highest energy level c. Electrons in the nucleus d. Electrons that have a low energy level in the cloud 47. What is a period? a. The columns on the periodic table that have atoms with the same properties b. The columns on the periodic table that have atoms with decreasing radaii c. The rows on the periodic table that have decreasing number of protons d. The rows on the periodic table that have atoms with increasing atomic number 48. What is a group? a. The columns on the periodic table that have atoms with the same properties b. The columns on the periodic table that have atoms with decreasing radaii c. The rows on the periodic table that have decreasing number of protons d. The rows on the periodic table that have atoms with increasing atomic number 49. Why is group 18 special? a. They are all metals b. They have all their valence electrons filled up and are stable c. They bond very easily with everything d. They are metalloids 50. How many valence electrons does group 2 have? a. 2 b. 4 Lesson 6 c. 6 d. 8 51. How many valence electrons does group 16 have? a. 2 b. 4 c. 6 d. 8 52. What is the unit we use for atomic radius? a. Millimeters b. Centimeters c. Kilometers d. Picometers 53. What do we call an atom that has a positive or negative charge? a. Bond b. Ion c. Chromosome d. Base Pair Directions: Determine if each compound is a covalent bond or ionic bond (1 point each) 54. Sodium Bromide (NaBr) ___Covalent_________________ 55. Lithium oxide (Li2O) ___Covalent_________________ 56. Water (H2O) ____Ionic________________ 57. Table salt (NaCl) ___Covalent_________________ 58. Methane (CH4) ____Ionic________________ Directions: Label each atomic theory based on who discovered the model (1 point each) + + + + + + 0 21.Dalton 22.Tomson 23.Rutherford 24.Bohr 26. How did the atomic theory continue to change as new scientists investigated atoms? (2 points) ___added neutrons, electron cloud, nucleus, Lesson 6 Directions: Label parts of an element from the periodic table on the line (1 point each) 26. Atomic Number 6 27. Atomic Symbol C 28. Atomic Mass 12.011 Directions: Choose three atoms from the periodic table and draw a Bohr’s Model of each atom in the box (2 points each) 29. 31. 30. Lesson 6 36. Why do we use the Bohr’s model of atoms if the theory is no longer accepted? (2 points) ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ __________________ Directions: Using the following data table, graph the atomic radaii (6 points) Atomic Number 4 12 20 38 56 Element Be Mg Ca Sr Ba Radius (in picometers) 112 160 197 215 222 Lesson 6 37. Do the points curve or stay in a straight line? (1 point) ________________________________________________________________________ _ 38. What does the trend show in relation to the size of atoms? (2 points) ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ __________________ 39. How does this relate to other groups on the periodic table? (2 points) ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ __________________ Directions: Draw electron dot diagram for each of the following elements (1 point each) 40. Li 41. P 42. Ca Directions: Draw electron dot diagrams for each of the following compounds. Label as ionic or covalent. (2 points each) 43. O2 44. AlF3