- Chemistry
... (a) [Co(H2O)6]2+(aq) + 4 Cl−(aq) → [CoCl4]2−(aq) + 6 H2O(l) Lewis base, Cl−, competes successfully with the Lewis base, H2O, to complex Co2+. (b) [Fe(H2O)6]2+(aq) + 6 CN−(aq) → [Fe(CN)6]4−(aq) + 6 H2O(l) Lewis base, CN−, competes successfully with the Lewis base, H2O, to complex Fe2+. (c) [Ni(H2O)6] ...
... (a) [Co(H2O)6]2+(aq) + 4 Cl−(aq) → [CoCl4]2−(aq) + 6 H2O(l) Lewis base, Cl−, competes successfully with the Lewis base, H2O, to complex Co2+. (b) [Fe(H2O)6]2+(aq) + 6 CN−(aq) → [Fe(CN)6]4−(aq) + 6 H2O(l) Lewis base, CN−, competes successfully with the Lewis base, H2O, to complex Fe2+. (c) [Ni(H2O)6] ...
Quantum properties of atomic-sized conductors
... thus form conducting nanowires. During the last stages of the pulling a neck-shaped wire connects the two electrodes, the diameter of which is reduced to single atom upon further stretching. For some metals it is even possible to form a chain of individual atoms in this fashion. Although the atomic ...
... thus form conducting nanowires. During the last stages of the pulling a neck-shaped wire connects the two electrodes, the diameter of which is reduced to single atom upon further stretching. For some metals it is even possible to form a chain of individual atoms in this fashion. Although the atomic ...
Schaum`s Outline of Theory and Problems of
... quantity of mass accounted for by the energy contained in a material object is so small that it is not measurable. Hence, the mass of an object is very nearly identical to the quantity of matter in the object. Particles of energy have very small masses despite having no matter whatsoever; that is, a ...
... quantity of mass accounted for by the energy contained in a material object is so small that it is not measurable. Hence, the mass of an object is very nearly identical to the quantity of matter in the object. Particles of energy have very small masses despite having no matter whatsoever; that is, a ...
UNIT 1. SOME BASIC CONCEPTS OF CHEMISTRY Concept
... Amount of Fe2O3 can be increased by taking any one of the reactants (iron or oxygen) in excess. Amount of Fe2O3 produced will decreases it the amount of any one of the reactants (iron or oxygen) is taken in excess. ...
... Amount of Fe2O3 can be increased by taking any one of the reactants (iron or oxygen) in excess. Amount of Fe2O3 produced will decreases it the amount of any one of the reactants (iron or oxygen) is taken in excess. ...
Anexo I. Modelos teóricos de análisis de sistemas de valencia mixta.
... intervalencia a altura mitad. En estas expresiones se ha asumido que la banda tiene forma Gaussiana. La energía de reorganización total, λ, puede expresarse como la suma de dos contribuciones independientes: la reorganización de los modos nucleares interiores, es decir, la alteración interna de la l ...
... intervalencia a altura mitad. En estas expresiones se ha asumido que la banda tiene forma Gaussiana. La energía de reorganización total, λ, puede expresarse como la suma de dos contribuciones independientes: la reorganización de los modos nucleares interiores, es decir, la alteración interna de la l ...
Quantum Wavepacket Dynamics in Molecular and Trapped Ion Systems Dong Wang
... interference can also take place between two wavepackets prepared by two pulses in the same electronic states, such as the fluorescence-detected wavepacket interferometry experiment in I2 by Scherer et al. [21], the photodissociation experiment in Cs2 by Girard et al. [22] and photodissociation exp ...
... interference can also take place between two wavepackets prepared by two pulses in the same electronic states, such as the fluorescence-detected wavepacket interferometry experiment in I2 by Scherer et al. [21], the photodissociation experiment in Cs2 by Girard et al. [22] and photodissociation exp ...
Applied Physics Letters
... field in ambient conditions.1,16 The experimental measurements find strong characteristics of CE enhancement for graphene. One possible explanation for the observed trend is that the changes in the electron density of graphene due to doping lead to a modulation of the local electric field at the sur ...
... field in ambient conditions.1,16 The experimental measurements find strong characteristics of CE enhancement for graphene. One possible explanation for the observed trend is that the changes in the electron density of graphene due to doping lead to a modulation of the local electric field at the sur ...
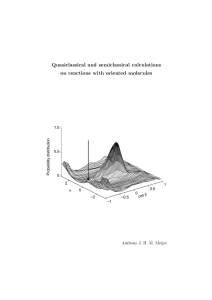
Quasiclassical and semiclassical calculations on reactions with oriented molecules cos β
... the Ba + N2 O reaction can be explained using the standard Angle Dependent Line of Centers (ADLC) model. In Chapter 2 a classical model is presented to explain an increasing steric e ect with increasing translational energy, as found e.g., for the Ca + CH3 F reaction. This so called \trapping model" ...
... the Ba + N2 O reaction can be explained using the standard Angle Dependent Line of Centers (ADLC) model. In Chapter 2 a classical model is presented to explain an increasing steric e ect with increasing translational energy, as found e.g., for the Ca + CH3 F reaction. This so called \trapping model" ...
Hyperfine Structure of Cs2 Molecules in Electronically Excited States
... tool for investigations. For instance, the combination of ultracold atoms and FR makes it possible to study properties of three-body Efimov states [33]. In our experiment, FRs play a leading role. We use a FR to create Feshbach molecules from a Cs BEC. Another important tool for quantum gas control ...
... tool for investigations. For instance, the combination of ultracold atoms and FR makes it possible to study properties of three-body Efimov states [33]. In our experiment, FRs play a leading role. We use a FR to create Feshbach molecules from a Cs BEC. Another important tool for quantum gas control ...
IB Chemistry Online SAQ_Ans
... d Main energy level 2 (second shell) i.e. n = 2 e Each element has its own characteristic line spectrum. Therefore an element can be identified by its line spectrum just as a criminal can be identified from a fingerprint. f An unknown yellow emission line was observed in the solar spectrum during ...
... d Main energy level 2 (second shell) i.e. n = 2 e Each element has its own characteristic line spectrum. Therefore an element can be identified by its line spectrum just as a criminal can be identified from a fingerprint. f An unknown yellow emission line was observed in the solar spectrum during ...
Slides - nanoHUB.org
... If we can make the average velocity go smoothly from the low VD to the high VD limit, then we will have a smooth model for ID(VG, VD). ...
... If we can make the average velocity go smoothly from the low VD to the high VD limit, then we will have a smooth model for ID(VG, VD). ...
New frontiers in quantum cascade lasers
... enough energy at the steps to create an electron–hole pair by impact ionization at the potential step given by band discontinuity. The number of stages typically ranges from 20 to 35 for lasers designed to emit in the 4–8 μm range, but working lasers can have as few as one or as many as over 100 sta ...
... enough energy at the steps to create an electron–hole pair by impact ionization at the potential step given by band discontinuity. The number of stages typically ranges from 20 to 35 for lasers designed to emit in the 4–8 μm range, but working lasers can have as few as one or as many as over 100 sta ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.