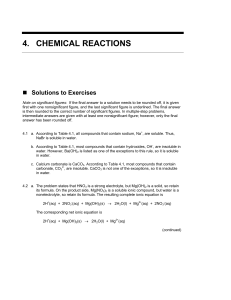
chapter 4 types of chemical reactions and solution stoichiometry
... unequal sharing of electrons in bonds that results in unequal charge distribution in the overall molecule. Polar molecules have a partial negative end and a partial positive end. These are not full charges as in ionic compounds but are charges much smaller in magnitude. Water is a polar molecule and ...
... unequal sharing of electrons in bonds that results in unequal charge distribution in the overall molecule. Polar molecules have a partial negative end and a partial positive end. These are not full charges as in ionic compounds but are charges much smaller in magnitude. Water is a polar molecule and ...
chapter 4 types of chemical reactions and solution stoichiometry
... unequal sharing of electrons in bonds that results in unequal charge distribution in the overall molecule. Polar molecules have a partial negative end and a partial positive end. These are not full charges as in ionic compounds but are charges much smaller in magnitude. Water is a polar molecule and ...
... unequal sharing of electrons in bonds that results in unequal charge distribution in the overall molecule. Polar molecules have a partial negative end and a partial positive end. These are not full charges as in ionic compounds but are charges much smaller in magnitude. Water is a polar molecule and ...
A Review of Surface Analysis Techniques for the
... pharmaceuticals, clean fuels, etc., as well as pollution abatement technologies, have a common catalytic origin. As catalysis proceeds at the surface, it is of paramount importance to gain insight into the fundamental understanding of local surface chemistry, which in turn governs the catalytic perf ...
... pharmaceuticals, clean fuels, etc., as well as pollution abatement technologies, have a common catalytic origin. As catalysis proceeds at the surface, it is of paramount importance to gain insight into the fundamental understanding of local surface chemistry, which in turn governs the catalytic perf ...
Synthetic Organic Chemistry - Name
... Due to the presence of bulky groups in both or OH either of the reactants in Grignard reagent the extent of addition is reduced or the reaction not take place or some abnormal product is formed. If Grignard reagent has β-hydrogen atom, then in some hindred ketone also show reduction of carbonyl grou ...
... Due to the presence of bulky groups in both or OH either of the reactants in Grignard reagent the extent of addition is reduced or the reaction not take place or some abnormal product is formed. If Grignard reagent has β-hydrogen atom, then in some hindred ketone also show reduction of carbonyl grou ...
Chemical Redox Agents for Organometallic
... solutions, the usual media for organometallic ET reactions. It is hoped that the information in this review will also be useful to those interested in ET reactions of organic and inorganic systems, which have more often involved the use of labile metal ions such as M(III), M ) Fe, Tl1 (but see ref 2 ...
... solutions, the usual media for organometallic ET reactions. It is hoped that the information in this review will also be useful to those interested in ET reactions of organic and inorganic systems, which have more often involved the use of labile metal ions such as M(III), M ) Fe, Tl1 (but see ref 2 ...
Chemical Redox Agents for Organometallic
... solutions, the usual media for organometallic ET reactions. It is hoped that the information in this review will also be useful to those interested in ET reactions of organic and inorganic systems, which have more often involved the use of labile metal ions such as M(III), M ) Fe, Tl1 (but see ref 2 ...
... solutions, the usual media for organometallic ET reactions. It is hoped that the information in this review will also be useful to those interested in ET reactions of organic and inorganic systems, which have more often involved the use of labile metal ions such as M(III), M ) Fe, Tl1 (but see ref 2 ...
SCH 206
... To the Student • This course is very wide, almost to the point that without a proper strategy may appear overwhelming. However, it may be rendered manageable if the student recognizes the fact that most of the reactions of organic compounds are centered on functional groups and, more importantly, a ...
... To the Student • This course is very wide, almost to the point that without a proper strategy may appear overwhelming. However, it may be rendered manageable if the student recognizes the fact that most of the reactions of organic compounds are centered on functional groups and, more importantly, a ...
View - OhioLINK Electronic Theses and Dissertations Center
... NMnOx-Y(Cl) are hexagonal birnessite type (poorly ordered) layered structure MnO2. Raman scattering also showed that these manganese oxides on the zeolite surface are edge sharing MnO6 octahedra chains, as found in birnessite. XPS characterization showed that all samples had Mn valences as in birnes ...
... NMnOx-Y(Cl) are hexagonal birnessite type (poorly ordered) layered structure MnO2. Raman scattering also showed that these manganese oxides on the zeolite surface are edge sharing MnO6 octahedra chains, as found in birnessite. XPS characterization showed that all samples had Mn valences as in birnes ...
Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005
... Chemical nomenclature must evolve to reflect the needs of the community that makes use of it. In particular, nomenclature must be created to describe new compounds or classes of compounds; modified to resolve ambiguities which might arise; or clarified where there is confusion over the way in which nom ...
... Chemical nomenclature must evolve to reflect the needs of the community that makes use of it. In particular, nomenclature must be created to describe new compounds or classes of compounds; modified to resolve ambiguities which might arise; or clarified where there is confusion over the way in which nom ...
fundamental concept of transition and inner
... Mercury is the only metal amongst transition elements which is liquid at 0oC due to weak metallic bond. (e) Transition metals show variable oxidation states due to participation of ns and (n-1) d electrons in bonding. (f) First ionization energy of 5d elements is higher than those of 3d and 4d eleme ...
... Mercury is the only metal amongst transition elements which is liquid at 0oC due to weak metallic bond. (e) Transition metals show variable oxidation states due to participation of ns and (n-1) d electrons in bonding. (f) First ionization energy of 5d elements is higher than those of 3d and 4d eleme ...
Hypervalent Iodine Reagents in High Valent Transition Metal
... Over the last 20 years, high valent metal complexes have transitioned from mere curiosities to being at the forefront of modern catalytic method development. This approach has enabled transformations complimentary to those possible via traditional manifolds, most prominently carbon-heteroatom bond f ...
... Over the last 20 years, high valent metal complexes have transitioned from mere curiosities to being at the forefront of modern catalytic method development. This approach has enabled transformations complimentary to those possible via traditional manifolds, most prominently carbon-heteroatom bond f ...