File
... Hydrazine also can hydrogen bond because it has covalent NH bonds as well as having a lone pair of electrons on each N. The high boiling point for hydrazine’s relatively small size supports this. N2(g) + 3 H2(g) ⇌ 2 NH3(g) + heat a. This reaction is exothermic, so an increase in temperature will de ...
... Hydrazine also can hydrogen bond because it has covalent NH bonds as well as having a lone pair of electrons on each N. The high boiling point for hydrazine’s relatively small size supports this. N2(g) + 3 H2(g) ⇌ 2 NH3(g) + heat a. This reaction is exothermic, so an increase in temperature will de ...
c00kieee - Ritter Illustration
... overall annual radiation exposure for people working in a synthetic or analytical radiological laboratory is less than that found for aircraft crew. In order to fully mitigate the risks of handling radioactive material and maintain a minimum radiation fields, it is important to have an understanding ...
... overall annual radiation exposure for people working in a synthetic or analytical radiological laboratory is less than that found for aircraft crew. In order to fully mitigate the risks of handling radioactive material and maintain a minimum radiation fields, it is important to have an understanding ...
The Influence of Base Metal (M) Oxidation State in Au-M
... The activity of the co-deposited fresh catalysts, measured at 35 °C (Figure 4) is generally much lower than that of the catalysts prepared by sequential deposition. The copper promoted catalyst surpasses the rest of that series, showing a higher conversion of 20%. The conversions measured at higher ...
... The activity of the co-deposited fresh catalysts, measured at 35 °C (Figure 4) is generally much lower than that of the catalysts prepared by sequential deposition. The copper promoted catalyst surpasses the rest of that series, showing a higher conversion of 20%. The conversions measured at higher ...
Chapter 8 "Ionic versus Covalent Bonding"
... 1. Atoms interact with one another to form aggregates such as molecules, compounds, and crystals because doing so lowers the total energy of the system; that is, the aggregates are more stable than the isolated atoms. 2. Energy is required to dissociate bonded atoms or ions into isolated atoms or io ...
... 1. Atoms interact with one another to form aggregates such as molecules, compounds, and crystals because doing so lowers the total energy of the system; that is, the aggregates are more stable than the isolated atoms. 2. Energy is required to dissociate bonded atoms or ions into isolated atoms or io ...
reactions of some transition metal ions
... Aqueous solutions contain the yellow-green, octahedral hexaaquairon(III) ion. It behaves as a typical M3+ ion. [Fe(H2O)6]3+(aq) + 3OH¯(aq) ...
... Aqueous solutions contain the yellow-green, octahedral hexaaquairon(III) ion. It behaves as a typical M3+ ion. [Fe(H2O)6]3+(aq) + 3OH¯(aq) ...
Ruthenium(II/III) - Publications of the IAS Fellows
... spectrometer fitted with a quartz dewar for measurements at 77 K (liquid nitrogen). The spectrum was calibrated by using tetracyanoethylene (tcne, g52.0023). The elemental analyses were carried out with a Carlo Erba (Italy) elemental analyzer. The following Hammett s values for p-substituents were u ...
... spectrometer fitted with a quartz dewar for measurements at 77 K (liquid nitrogen). The spectrum was calibrated by using tetracyanoethylene (tcne, g52.0023). The elemental analyses were carried out with a Carlo Erba (Italy) elemental analyzer. The following Hammett s values for p-substituents were u ...
Comparison of structurally-related alkoxide, amine, and thiolate
... 1.20 mdynes/cm) well-below that of any other reported iron-peroxo, thereby favoring Fe–O bond cleavage and H2O2 release [24]. Superoxide reductase, peptide deformylase, and nitrile hydratase occupy an unique niche amongst known non-heme enzymes in that they contain cysteinate ligands, as opposed to ...
... 1.20 mdynes/cm) well-below that of any other reported iron-peroxo, thereby favoring Fe–O bond cleavage and H2O2 release [24]. Superoxide reductase, peptide deformylase, and nitrile hydratase occupy an unique niche amongst known non-heme enzymes in that they contain cysteinate ligands, as opposed to ...
Oxygenation of saturated and unsaturated hydrocarbons with
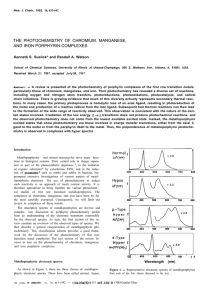
... oxo-manganese species should bear (IV) or (V) oxidation state, depending on the mechanism of oxidation reactions. To the best of our knowledge, formation of the oxo-manganese(V) is more reliable for NaIO4Mn(Por) oxidation system. The results of tables 1 and 2 allow us to modulate the observed substr ...
... oxo-manganese species should bear (IV) or (V) oxidation state, depending on the mechanism of oxidation reactions. To the best of our knowledge, formation of the oxo-manganese(V) is more reliable for NaIO4Mn(Por) oxidation system. The results of tables 1 and 2 allow us to modulate the observed substr ...
File - cpprashanths Chemistry
... 2. Question nos. 1 to 8 are very short answer questions and carry 1 mark each. 3. Question nos. 9 to 18 are short answer questions and carry 2 marks each. 4. Question nos. 19 to 27 are also short answer questions and carry 3 marks each 5. Question nos. 28 to 30 are long answer questions and carry 5 ...
... 2. Question nos. 1 to 8 are very short answer questions and carry 1 mark each. 3. Question nos. 9 to 18 are short answer questions and carry 2 marks each. 4. Question nos. 19 to 27 are also short answer questions and carry 3 marks each 5. Question nos. 28 to 30 are long answer questions and carry 5 ...