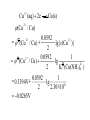
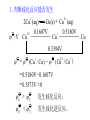
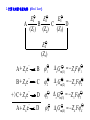
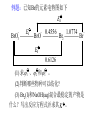
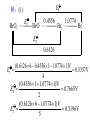
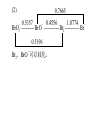
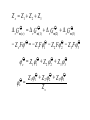* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 幻灯片 1
Jahn–Teller effect wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Process chemistry wikipedia , lookup
Oxidation state wikipedia , lookup
Biochemistry wikipedia , lookup
Safety data sheet wikipedia , lookup
Electrochemistry wikipedia , lookup
Livermorium wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Click chemistry wikipedia , lookup
Organic chemistry wikipedia , lookup
Bond valence method wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Periodic table wikipedia , lookup
Molecular orbital wikipedia , lookup
Institute of Chemistry Ceylon wikipedia , lookup
Bent's rule wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Coordination complex wikipedia , lookup
Atomic orbital wikipedia , lookup
Electronegativity wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Analytical chemistry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Green chemistry wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
History of chemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Metallic bonding wikipedia , lookup
Nuclear chemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
Extended periodic table wikipedia , lookup
Computational chemistry wikipedia , lookup
Chemical bond wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Electron configuration wikipedia , lookup
Atomic theory wikipedia , lookup
INORGANIC CHEMISTRY Teachers: Prof. Zhang Wei-guang (章伟光) Tel: 13416250262(HP), E-mail: [email protected] Dr. Fan Jun(范军) Tel: 85210267(H), 85210875(o); E-mail: [email protected] Sept. 2005 For Science Class 2 INORGANIC CHEMISTRY Contents Contents Introduction Section A Atomic structure Section B Introduction to inorganic substance Section C Structure and bonding in molecules Section D Structure and bonding in solids INORGANIC CHEMISTRY Contents Section E Chemistry in solution Section F Chemistry in nonmetals Section G Non-transition metals Section H Chemistry of transition metals Section I Lanthanides and actinides Section J Environmental, biological and industrial aspects INORGANIC CHEMISTRY Introduction Introduction 1. Emergence and Development of Chemistry Pride of Chemist Four Inventions in ancient China and Alchemy in ancient Greece Nature phenomenon: Burning, Volcano breaking out, and Lightning, etc. High technology production in modern civilization Set up theoretic system of modern chemistry and industry INORGANIC CHEMISTRY Introduction 2. Brach of Chemistry CHEMISTRY Inorganic Chemistry Coordinary Chemistry Organic Chemistry Polymer Chemistry Analytical Chemistry Organometallic Chemistry Electro-Chemistry Physical Chemistry Structural Chemistry …… Biochemistry Geochemistry Spacechemistry Material Chemistry Nano-Chemistry etc. INORGANIC CHEMISTRY Introduction 3. Ways of Learning Chemistry Study hard Learn cleverly Do it yourself INORGANIC CHEMISTRY Introduction 4. Some basic conception in Chemistry Nucleus reaction Protons + Neutrons = Nucleus Breaking Atoms Molecules Forming Get or Lost Electrons Chemical reaction INORGANIC CHEMISTRY Atom Introduction Nucleus + Electrons Removal (addition) of Protons or Neutrons Removal (addition) of electrons Isotopes, New Element Ions, Free radical Isotopes are atoms with the same atomic number but different numbers of the neutrons for one element, so their mass numbers will be different. They have the same amount of protons. Potential application: NMR, dating and origin of rocks, the studies for the reaction mechanism, etc INORGANIC CHEMISTRY Introduction Gas Species discussed in this course Liquid, Solution Solid n-----mol Molar mass-----gmol-1 Molar volume---- m3mol-1 lmol-1, dm3mol-1 SI units: Pressure, P-----Pa, KPa, atm Temperature, T-----K, ℃ Energy----- J, or kJ, cal, kcal Gas INORGANIC CHEMISTRY 1. Ideal-gas Law No intermolecular interaction Ideal gas The volume of the molecules in gas would be considered as zero. GAS Real gas This content would be introduced in Physics and Physical Chemistry . (1) Ideal-gas equation For the mono-component ideal-gas P——pressure (Pa); V ——volume (m3); n —— moles (mol); T ——temperature (K) T= t + 273.15; d —— density (gmol-1) PV= n R T d RT P M (i) If SI units would be used, R = 8.314 Jmol-1K-1; (ii) P—KPa, V—dm3, T—K, n—mol, R = 8.314 J mol-1 K-1; (ii) P—atm, V—l, T—K, n—mol, R = 0.08206 atm l mol-1 K-1 INORGANIC CHEMISTRY Introduction For example: (a) 现在一般能得到的最高真空是10-12托(1托=1 mmHg)。求27℃下体积为1ml的真 空体系中的气体分子数。 PV=nRT n = PV/(RT) = 5×10-20 mol N= n×6.023×1023 = 3×104 个 (b) 空气的N2和O2的体积比例分别为79%与21%,计算空气在25℃和100KPa下空 气的密度是多少?(gdm-3) d RT P M d = 1.16 gdm-3 M =28×79%+32×21%= 28.8 INORGANIC CHEMISTRY Introduction (2) Law of partial pressure For the Poly-component ideal-gas If the chemical reaction don’t take place among the component, the total moles would keep constant after mixed them. If V=0 and T=0, PT Pi P1 P2 P3 .... nT ni n1 n2 n3 .... RT V n PiV ni RT PT V nT RT xi ni nT xi 1 volumefraction Mole fraction Vi VT i partial pressure of one kind of component Gas INORGANIC CHEMISTRY For example: (1) 将730mmHg 压力的氮气60ml,300mmHg 压力的氧气200 ml及420mmHg压力的 氢气80ml 压入250ml 的真空瓶中,求混合后的气体分压力和混合的总压力。 PT Pi P1 P2 P3 .... RT V n i PiV ni RT PN2= 175mmHg, PO2=240mmHg, PH2=134mmHg, PT = 549mmHg (2) 某 容 器 中 含 有 NH3 、 O2 、 N2 等 气 体 的 混 合 物 。 取 样 分 析 后 , 其 中 n(NH3)=0.320mol , n(O2)=0.180mol , n(N2)=0.700mol 。 混 合 气 体 的 总 压 p=133.0kPa。试计算各组分气体的分压。 n= n(NH3)+n(O2)+n(N2) nB pB p xB p n gas INORGANIC CHEMISTRY (3) Diffusion Law of gas u1 d1 M2 u2 d2 M1 u--the diffusion rate of the gas molecules d--the density of the gas M--the mole mass of the gas (4) Real gas Homework: 1, 3, 7 INORGANIC CHEMISTRY Section A A1 Section A1 Atomic structure THE NUCLEAR ATOM Electron and Nuclei An atom consists of a very small positively charged nucleus, surrounded by negative electrons held by electrostatic attraction. The motion of electrons changes when chemical bonds are formed, nuclei being unaltered. Nucleus: positive charge Electron: negative charge Electrostatic attractive force: Electrons are attracted to Nucleus. Electrostatic repulsion force: among the protons. From planetary model to quantum theory Section A1 INORGANIC CHEMISTRY Nuclear structure Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. Electrostatic attractive force: Electrons are attracted to Nucleus The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Electrostatic repulsion force: among the protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. Magic numbers: 2 8 20 28 50 82 126 16(8+8)O 208(82+126)Pb INORGANIC CHEMISTRY Section A1 Isotopes Isotopes are atoms with the same atomic number but different numbers of neutrons. Many elements consist naturally of mixtures of isotopes, with very similar chemical properties. Only one stable isotopes: 9Be, 19F, 23Na, 27Al, 31P, Sc, Mn, etc. total numbers: 20. Several stable isotopes: 1H, Deuterium: 2H, 3H; Tin (Sn): no fewer than 10. 12C, 13C, 14C; etc. Relative atomic mass (RAM) is equal to molar mass of element RAM of an element: RAM ( f i Mri ) fi: the abundance of the isotopes in the nature; Mri: the relative atomic number of the isotopes INORGANIC CHEMISTRY Application: (1) NMR: 2H, 13C, nuclear magnetic resonance (2) Unstable radioactive: medical diagnostics (3) 14C: judge years; (4) investigate the detailed mechanism of chemical reactions Nuclides (核素): Isotopes (同位素): Element (元素): Atom (原子): isotone (同中素): Isobar (同量异位素): Section A1 INORGANIC CHEMISTRY Section A1 Radioactivity: Radioactive decay: is a process whereby unstable nuclei change into more stable ones by emitting high-energy particles of different kinds. All elements with Z > 83 are radioactive. The process involved are as follows: (a) decay: 4He (b) decay: electron (c) decay: high-energy electromagnetic radiation. It often accompanies and decay. Half-life: INORGANIC CHEMISTRY A2 Section A2 ATOMIC ORBITALS Wavefunctions The quantum theory is necessary to describe electrons. It predicts discrete allowed energy levels and wavefunction, which give probability distributions for electrons. Wave functions for elections in atoms are called atomic orbitals. Quantum mechanics Quantized energy Probability distributions Schrödinger’s wave equation Atomic orbitals INORGANIC CHEMISTRY Quantum numbers and nomenclature A3 Section A2 A3 Atomic orbitals are labeled by three quantum numbers n,l and m. Orbitals are called s,p,d or f according to the value of 1; there are respectively one, three,five and seven different THE NUCLEAR ATOM possible m values for these orbitals. Electron and Nuclei Principal Quantumofnumbers An atom consists a very small positively charged nucleus, n=1, 2, 3….. surrounded by negative electrons held by electrostatic Angular momentum (or azimuthal) Quantum numbers attraction. The motion of electrons changes when chemical l=0, 1, 2, …, (n-1) bonds are formed, nuclei being unaltered. Magnetic Quantum numbers Nuclear structure m=0, ±1, ±2, …, ±(n-1)protons and uncharged neutrons. Nuclei contain positive Spin Quantum The number of numbers protons is the atomic number (Z) of an ms=+1/2, -1/2 element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Angular functions: ‘shapes’ A3 Section A2 A3 s orbitals are spherical. p orbitals have two directional lobes, which can point in three possible directions. d and f oritals have coorespondingly greater numbers of directional lobes. THE NUCLEAR ATOM Electron and Nuclei Nuclear structure Radial An atomwavefunction consists of a very small positively charged nucleus, surrounded by negative electrons held by electrostatic Angular attraction.wavefunction The motion of electrons changes when chemical bonds are formed, nuclei being unaltered. Polar diagrams Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an Boundary surface element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between Nodal plane protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Section A2 INORGANIC CHEMISTRY Radial distributions A3 Section A2 A3 The radial distribution function shows how far from the nucleus an election is likely to be found. The major features depend on n but there is some dependence on l. THE NUCLEAR ATOM Electron and Nuclei Radial probability distributions An atom consists of a very small positively charged nucleus, surrounded by negative electrons held by electrostatic attraction. The motion of electrons changes when chemical bonds are formed, nuclei being unaltered. Nuclear structure Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Section A2 INORGANIC CHEMISTRY Energies in hydrogen A3 Section A2 A3 The allowed energies in hydrogen depend on n only. They can be compared with experimental line spectra and the ionization THE NUCLEAR ATOMenergy. En= -R/n2 An atom consists of a very smalleVpositively Rydberg constant: R=13.595 per atom charged nucleus, Electron and Nuclei surrounded heldlimit by electrostatic When n=∞, Eby which is electrons the ionization ∞=0negative attraction. The motion Eof∞-E electrons changes when chemical Ionization energy: 1=R bonds are formed, nuclei being unaltered. Degenerate: Orbitals with the same n but different values of l and m have the same energy. Nuclear structure Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Hydrogenic ions A3 Section A2 Increasing nuclear charge in a one-electron ion leads to contraction of the orbital and an increase in binding energy of the electron. THE NUCLEAR ATOM For example: He+, Li2+ Electron and Nuclei <r>= n2aconsists An atom of a very small positively charged nucleus, 0/Z Where a0 is Bohr radius, theelectrons average radius of aelectrostatic 1S orbital in surrounded by negative held by hydrogen. attraction. The motion of electrons changes when chemical Z: atomic number bonds are formed, nuclei being unaltered. En= -Z2R/n2 Nuclear structure Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY A3 Section A3 MANY-ELECTRON ATOMS The orbital approximation Putting electrons into orbitals similar to those in the hydrogen atom gives a useful way of approximating the wavefunction of a many-electron atom. The electron configuration specifies the occupancy of orbitals, each of which has an associated energy. Orbital approximation Electron configuration Orbital energy INORGANIC CHEMISTRY Electron spin A3 Section A3 Electrons have an intrinsic rotation called spin, which may point in only two possible directions, specified by a quantum number ms. Two electrons in the same orbital with opposite THE NUCLEAR ATOM Electron and Nuclei spin are paired. Unpaired electrons give rise to paramagnetism. An atom consists of a very small positively charged nucleus, Spin Quantum numbers electrons held by electrostatic surrounded by negative m s=+1/2, -1/2 attraction. The motion of electrons changes when chemical bonds are formed, nuclei being unaltered. Nuclear structure Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Pauli exclusion principle A3 Section A3 When the spin quantum number m, is included no two electrons in an atom may have the same set of quantum numbers. THE NUCLEAR ATOMThus a maximum of two electrons can occupy any orbital. Electron and Nuclei Nuclear structure An atom consists of a very small positively charged nucleus, Li : (1s)2 (2s) surrounded by1 negative electrons held by electrostatic attraction. The motion of electrons changes when chemical bonds are formed, nuclei being unaltered. Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Effective nuclear charge A3 Section A3 The electrostatic repulsion between electrons weakens their binding in an atom, this is known as screening or shielding. TheATOM combined effect of attraction to the nucleus and THE NUCLEAR Electron and Nuclei Nuclear structure repulsion from other electrons is incorporated into an effective nuclear charge. An atom consists of a very small positively charged nucleus, surrounded by negative electrons held by electrostatic 2R/n2 E attraction. motion of electrons changes when chemical n= -Zeff The bonds are formed, nuclei being unaltered. σ = Z-Zeff Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Screening and penetration A3 Section A3 An orbital is screened more effectively if its radial distribution does not penetrate those of other electrons. For a given n, s orbitals are least screened and have the lowest THE NUCLEAR ATOM energy; p, d,... orbitals have successively higher energy. Electron and Nuclei Nuclear structure An atom consists of a very small positively charged nucleus, Degenerate surrounded by negative electrons held by electrostatic attraction. The motion of electrons changes when chemical Penetrating bonds are formed, nuclei being unaltered. Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Hund’s first rule A3 Section A3 When filling orbitals with l>0, the lowest energy state is formed by putting electrons so far as possible in orbitals with different m values, and with parallel spin. THE NUCLEAR ATOM Electron and Nuclei Nuclear structure An atom consists of a very small positively charged nucleus, surrounded by negative electrons held by electrostatic attraction. The motion of electrons changes when chemical bonds are formed, nuclei being unaltered. Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY A4 History Section A4 THE PERIODIC TABLE The periodic table - with elements arranged horizontally in periods and vertically in groups according to their chemical similarity - was developed in an empirical way in the 19th century. A more rigorous foundation came, first with the use of spectroscopy to determine atomic number and, second with the development of the quantum theory of atomic structure. INORGANIC CHEMISTRY Section A4 Building up The 'aufbau' or 'building up' principle gives a systematic method for determining the electron configurations of atoms and hence the structure of the periodic table. A3 THE NUCLEAR ATOM Elements in the same group have the same configurati0n of outer electrons. The way different orbitals are filled is controlled by their energies (and hence their An atom consists of a very small positively charged nucleus, Electron and Nuclei different screening by other electrons) and by the Pauli exclusion principle. surrounded by negative electrons held by electrostatic attraction. The motion of electrons changes when chemical According to the aufbau principle, the ground-state electron configuration of bonds are formed, nuclei being unaltered. an atom can be found by putting electrons in orbitals, starting with that of Nuclei contain positive protons and uncharged neutrons. lowest energy and moving progressively to higher energy. Nuclear structure (1) the lowest energyThe number of protons is the atomic number (Z) of an (2)Pauling exclusionelement. principleThe attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between (3)Hund’s rule protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY A3 Section A4 Penetration effect THE NUCLEAR ATOM Electron and Nuclei Nuclear structure Screening effect. Intervening of An atom consists of a very small positively charged nucleus, energy level surrounded by negative electrons held by electrostatic attraction. The motion of electrons changes when chemical bonds are formed, nuclei being unaltered. Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Electron configuration: The outmost electron configuration: Valence electron configuration: Section A4 INORGANIC CHEMISTRY Block structure A3 Section A4 The table divides naturally into s, p, d and f blocks according to the outer electron configurations. s and p blocks form the main groups, the d block the transition elements, and the f block the lanthanides and actinides. THE NUCLEAR ATOM Electron and Nuclei Nuclear structure An atom consists of a very small positively charged nucleus, surrounded by negative electrons held by electrostatic attraction. The motion of electrons changes when chemical bonds are formed, nuclei being unaltered. Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Section A4 Group numbers and names Modern group numbering runs from 1 to 18, with the j bl0cks being subsumed into groupTHE 3. Older (and contradictory) A3 NUCLEAR ATOM numbering systems are still found. Some groups of elements are conventionally given names, the most commonly used being alkali metals (group l), alkaline halogens (17) small and noble gases charged (18). nucleus, Anearths atom (2), consists of a very positively Electron and Nuclei Alkali metal : Alkaline metal: Transition metal: Nuclear structure Coinage metal: Chalcogen: Halogen: Noble gas: surrounded by negative electrons held by electrostatic attraction.rare Theearth: motion of electrons changes when chemical bonds arelanthanide: formed, nuclei being unaltered. actinide: Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Section A5 A5 TRENDS IN ATOMIC PROPERTIES Energy and sizes Trends in orbital energy and size reflect changes in the principal quantum number and effective nuclear charge. They are seen experimentally in trends in ionization energy (IE) and apparent radius of atoms. From: En= -Zeff2R/n2 Ionization energy: E∞-E1=R To Average radius <r>=n2a0/Zeff Metallic radii: Covalent radii: Ionic radii: Van der Waals’ radii: INORGANIC CHEMISTRY Horizontal trends A3 Section A5 Increasing nuclear charge causes a general increase of IE and a decrease of radius across any period. Breaks in the IE bend are found following the complete or half filling of any set of orbitals. THE NUCLEAR ATOM Electron and Nuclei Nuclear structure An atom consists of a very small positively charged nucleus, surrounded by negative electrons held by electrostatic attraction. The motion of electrons changes when chemical bonds are formed, nuclei being unaltered. Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Section A5 Vertical trends A3 THEANUCLEAR ATOM general increase of radius and decrease in IE down most groups is dominated by the increasing principal quantum An atom consistsEffective of a very nuclear small positively nucleus, Electron and number Nuclei of outer orbitals. charge charged also by to negative electrons heldIEbytrends. electrostatic increases, andsurrounded can give rise irregularities in the attraction. The motion of electrons changes when chemical bonds are formed, nuclei being unaltered. Nuclear structure Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Section A5 States of ionization A3 IEs for positive ions always increase with the charge. THE NUCLEAR ATOM M2+, M3+, …., second, third,…. consists of a very small positively charged nucleus, Electron and NucleiElementsAn atom IE IE IE 1 2 3 surrounded by negative electrons held by electrostatic Be (1s22s2) 9.3ev 18.2ev 154ev attraction. The motion of electrons changes when chemical bonds 6.1ev are formed, nuclei51ev being unaltered. Ca (Ar)4s2 11.9ev Nuclear structure Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Section A5 Electron affinity of an atom: defined as the ionization energy of the negative ion, thus the energy input in the process: M- M + e- INORGANIC CHEMISTRY Section B Section B1 Introduction to inorganic substance B1 ELECTRONEGATIVITY AND BOND TYPE Definition Electronegativity is the power of an atom to attract electrons to itself in a chemical bond. Elements of low electronegativity are called electropositive. Pauling Electronegativity: based on bond energies Mulliken Electronegativity: the average of the first ionization energy and the electron affinity of an atom. Allred-Rochow Electronegativity: proportional to Zeff/r2. INORGANIC CHEMISTRY Section B1 Different numerical estimates agree on qualitative trends: Electronegativity increases from left to right along a period, and generally decreases groups in the periodic table. INORGANIC CHEMISTRY Section A3 B1 The bonding triangle Electronegativity elements form meta1lic solids. Electronegative elements form or polymericATOM solids with covalent bonds. Elements of very different A3molecules THE NUCLEAR electronegativity combine to form solids that can be described by the ionic model. An atom consists of a very small positively charged nucleus, Delocalization of electrons: surrounded by negative electrons held by electrostatic Covalent compounds: attraction. The motion of electrons changes when chemical Giant covalent lattices (Polymeric solids): bonds are formed, nuclei being unaltered. Localized bonds: Nuclei contain positive protons and uncharged neutrons. Nuclear structure Cation: The number of protons is the atomic number (Z) of an Anion: element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. Electron and Nuclei INORGANIC CHEMISTRY Section B1 INORGANIC CHEMISTRY Bond polarity A3 Section A3 B1 The polarity of a bond arises from the unequal sharing of electrons between atoms with different electronegativities. There is no sharp dividing line between polar covalent and ionic substances. THE NUCLEAR ATOM Electron and Nuclei Nuclear structure Homopolar (A covalent between two atoms of the same ELs) An atom consists of a very small positively charged nucleus, Bond polarity surrounded by negative electrons held by electrostatic Heteropolar (A covalent when between two atoms of the different ELs attraction. The motion of electrons changes chemical bonds are formed, nuclei being unaltered. Electric dipolepositive momentprotons and uncharged neutrons. Nuclei contain The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY B2 Section B2 CHEMICAL PERIODICITY Introduction Major chemical trends, horizontally and vertically in the periodic table, can be understood in terms of changing atomic properties. This procedure has its limitations and many details of the chemistry of individual elements cannot be predicted by simple interpolation from their neighbors. INORGANIC CHEMISTRY Section A3 B2 Metallic and nonmetallic elements A3 THE NUCLEAR ATOM Metallic elements are electropositive, form electrically An atom consists of a very small positively charged nucleus, Electron and Nuclei conducting solids and have Non-metallic surrounded by cationic negative chemistry. electrons held by electrostatic elements, found in the upper right-hand portion ofchanges the periodic attraction. The motion of electrons when chemical table, have predominantly cova1ent andbeing anionic chemistry. The bonds are formed, nuclei unaltered. chemical trend is continuous and elements on the borderline Nuclei contain positive protons and uncharged neutrons. show intermediate characteristics. Nuclear structure The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Horizontal trends A3 Section A3 B2 Moving to the right in the periodic table, bonding character changes as electronegativity increases. The increasing number of ATOM electrons in the valence shell also gives rise to changes in THE NUCLEAR Electron and Nuclei Nuclear structure the stoichiometry and structure of compounds. Similar trends operate the d block. An atominconsists of a very small positively charged nucleus, surrounded by negative electrons held by electrostatic Closed shell attraction. The motion of electrons changes when chemical Lattice energy bonds are formed, nuclei being unaltered. Octet rule Nuclei contain positive protons and uncharged neutrons. Non-bonding electrons The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Section B3 B3 STABILITY AND REACTIVITY Introduction Stability and reactivity can be controlled by thermodynamic factors (depending only on the initial and final states and not on the reaction pathway) or kinetic ones (very dependent on the reaction pathway). Both factors depend on the conditions, and on the possibility of different routes to decomposition or reaction. Thermodynamic stable:stable or unstable Kinetic stable :reactive or unreactive INORGANIC CHEMISTRY Section B3 1. Very important concepts of thermodynamics: (1) System: the objects in research: (a) closed, (b) open, (c) isolated system (2) Surroundings: the others except for the system (3) State: the total parameters used to describe the physical and chemical properties of the system. The parameters P, V, T, n would be needed for the state of gas。 (4) process: the route from the initial state to final state. isothermal process (T=0); isobaric process (P=0); isovolumic process (V=0); adiabatic process ( Q=0). (5) State function: From state A to state B, some processes were adopted, but the total value for any process would be keep constant. state A —— state B—— State A, = 0 INORGANIC CHEMISTRY Section B3 (6) Heat (Q): the energies related with the change of temperature. positive Q: the system would absorb the energy from the circumstance; negative Q: release the energy from the system. (7) Work (W) : the others energies except for the heat. positive W: negative W: the volume work W = (PV) (8) Intrinsic energy (U) (state function): the total energy of the object in research. U En if from State A (U1)—— state B(U2), U1 = U2- U1 if from State B (U2)—— state A(U1), U2 = U1- U2 U1 + U2 = 0. INORGANIC CHEMISTRY Section B3 2. The fist law of thermodynamics (the law of conservation of energy ) for a closed system, U = Q - W 3. Enthalpy (焓) if P =0, U=U2-U1 = Q - W = (Q2- Q1) - (P2V2-P1V1) Qp = (U2+P2V2)-(U1+P1V1)=H2-H1= H defined as U+PV H (enthalpy)--state function So, (i) if P =0 , H =Qp +(positive), endothermic process - (negative), exothermic process (ii) if V =0 , U =QV Qp= QV + n RT INORGANIC CHEMISTRY Hess’ Law Section B3 Enthalpy change (△H) is the heat input to a reaction, a useful measure of the energy change involved. As △H does not depend on the reaction pathway (Hess' Law) it is often possib1e to construct thermodynamic cycles that allow va1ues to be estimated for processes that are not experimentally accessible. Overall △H A3 THE NUCLEAR ATOM values for reactions can be calculated from tabulated enthalpies of formation. An atom consists of a very small positively charged nucleus, Electron and Nuclei surrounded by negative electrons held by electrostatic attraction. The motion of electrons changes when chemical bonds are formed, nuclei being unaltered. Nuclear structure Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Section B3 Standard state: 100kPa, 298.15K The standard enthalpy of formation (Hf0): H0f of any compounds is defined as the energy change from its most stable elementary A3 THE NUCLEAR substance at the standard state. ATOM C (graphite) + O2(g) CO2 (g) Hf0 (CO2) An atom consists of a very small positively charged nucleus, Electron and C (diamond) + Nuclei O2(g) CO2 (g) Hf0 (CO2) surrounded by negative electrons held by electrostatic 0 By definition, Hf is zero for any elements in its stable (standard) states. attraction. The motion of electrons changes when chemical Application: (Hess’ Law) bonds are formed, nuclei being unaltered. The standard enthalpy change (H0) in any reaction would be calculated by using Nuclei contain positive protons and uncharged neutrons. structure theNuclear standard enthalpy of formation. The number of protons is the atomic number (Z) of an For example, for this reaction element. The attractive strong interaction between protons a A (state) + b B (state) and c C neutrons (state) + is dD (state) by (thermochemical equation), opposed electrostatic repulsion between protons. Repulsion dominates H 0 i H ( formation ) k H (reac tan ts) as Z increases and there is f f only a limited number of stable elements. [c H f (C ) d H f ( D)] [ a H f ( A) b H f ( B )] INORGANIC CHEMISTRY Section B3 Example.: Calculate the standard enthalpy change (rHm) in the following reaction, respectively. 3 NO2 (g)+ H2O(l) 2 HNO3 (l) + NO (g) Answer.: The standard enthalpy of formation (Hf , kJmol-1) of these compound are listed in Table. NO2 (g) 33.18 kJmol-1 H2O(l) -285.83 kJmol-1 HNO3(l) -173.23 kJmol-1 NO(g) 90.25 kJmol-1 Applying Hess’ law: rHm = ifHm (product)- ifHm (reactant) rHm = [ 2 (-173.23) + 90.25] – [3 33.18 + (-285.83)] = -69.92 kJmol-1. INORGANIC CHEMISTRY Section B3 Entropy 1. Entropy(熵) is a measure of molecular disorder or more precisely ‘the number of microscopic arrangements of energy possible in a macroscopic sample’. A3 THE NUCLEAR (1) Entropy increases withATOM rise in temperature and depends strongly on the state. (2) S(gas) >>S (liquid) > S (solid) Entropy change (△S) are invariably for reactions generate gas An atom consists ofpositive a very small positively that charged nucleus, Electron and Nuclei molecules. surrounded by negative electrons held by electrostatic (3) molecule mass or itsattraction. structure: The motion of electrons changes when chemical 2. Entropy:——state function bonds are formed, nuclei being unaltered. Applying the Hess’ Law contain positive protons and uncharged neutrons. △Sreaction = ∑S(products) Nuclei - ∑S(reactants) Nuclear structure number change of protons is the (Z)direction of an for For an isolated system, The the entropy would be atomic used tonumber judge the spontaneous reaction. element. The attractive strong interaction between protons If △S >0, the forward reaction would be spontaneous. and neutrons is opposed by electrostatic repulsion between = 0, the reaction would keep the balance state. as Z increases and there is < 0, the backwardprotons. reactionRepulsion would bedominates spontaneous. a limited of stable elements. ———— theonly second law ofnumber thermodynamics INORGANIC CHEMISTRY Section B3 S: by definition: the standard entropy of the ideal crystal for any pure compounds would be considered as zero at 0 K. ——the third law of thermodynamics S0m (the standard entropy) in standard state: the related data(△H0f,,S0m) would be found in the appendix. For any reaction, a A (state) + b B (state) c C (state) + d D (state) △rSm0 (reaction)= ∑Sm0 (products) - ∑Sm0 (reactants) r H m i H f ( products) k H f (reac tan ts) [c H f (C ) d H f ( D)] [a H f ( A) b H f ( B)] INORGANIC CHEMISTRY Gibbs free energy(G) △ S —— the application were be confined for an isolated system. If △P=0 and △T=0, From the first law and the second law of thermodynamics, G = H -TS G: the Gibbs free energy, (state function) △G = △G (final state) -△G (initial state) If △G <0, the forward reaction would be spontaneous. = 0, the reaction would keep the balance state. > 0, the backward reaction would be spontaneous. Section B3 INORGANIC CHEMISTRY Section B3 △G0f of any compounds is defined as the Gibbs free energy change from its most stable elementary substance at the standard state. By definition, G0f is zero for any elements in its stable (standard) states. for this reaction a A (state) + b B (state) c C (state) + d D (state) Applying the Hess’ Law, G 0 i G f ( products) k G f (reac tan ts) [c G f (C ) d G f ( D)] [a G f ( A) b G f ( B)] INORGANIC CHEMISTRY Section B3 For example: CaCO3 (s) CaO(s) + CO2 (g) (1) At the Standard state, G0 = +130kJmol-1. However, (2) at 1273K, 100kPa, G = -25 kJmol-1 (3) at 1273K, 0.1kPa, G = -174 kJmol-1 G0f (standard state) If under non-standard state, how to obtain G(T) ? (1) Van’t Holf equation(范特霍夫等温方程): r Gm (T ) r Gm (T ) RT ln J J ( Pi i ) (气体反应体系) P or J ( Ci i ) (溶液反应体系) C P. T G INORGANIC CHEMISTRY Section B3 (2) 吉布斯-亥姆霍兹方程 G = H -TS G = H -T S G (T) = H0 -T S0 G (T) = H0(298K) -T S0(298K) If G = 0, the reaction would keep the balanced state. Tm(eq) = H0(298K) / S0(298K) H0(298K) S0(298K) G0(298K) Example - (exothermic) +(entropy increase) - (spontaneous) H2(g) + F2(g) 2 HF (g) + (endothermic) -(entropy decrease) +(not spontaneous) CO (g) C (s) + O2 (g) - (exothermic) - + or - NH3 + HCl NH4Cl + (endothermic) + + or - CaCO3 CaO + CO2 INORGANIC CHEMISTRY Section B3 Example. In general, the following course was applied in purification of coarse nickel: first, Ni(CO)4 was synthesized by the reaction of nickel and CO at 323K; then, decomposition of Ni(CO)4(l) would produce the high-purity nickel. Ni(s) + 4 CO (g) Ni(CO)4 (l) Try to analysis the rationality of this process from these data: rHm0 = -161 kJmol-1, rSm0 = -420 Jmol-1K-1. Answer. : Applying rGm= rHm – T rSm, the temperature at equilibrium (rGm) would be obtained. rGm (T) = 0 T = rHm / rSm = -161 103 / (-420) = 383 K. if T < 383 K, rGm < 0, the normal reaction will occur successfully. If T > 383K, rGm > 0, the reverse reaction will take place. So, at lower temperature, Ni(CO)4 was synthesized by the reaction of coarse nickel and CO. And, at higher temperature, the preparation of the high-purity nickel would be performed by decomposition of Ni(CO)4. INORGANIC CHEMISTRY Chemical Equilibrium (1) Dynamic equilibrium, or running balance A (state) B (state) forward reaction: Backward reaction: For example: Some NaCl was dissolved into the water at room temperature. Chemical equilibrium: the object: the reaction in the closed system Section B3 INORGANIC CHEMISTRY Section B3 (2) Equilibrium constants For a general reaction such as a A (state) + b B (state) c C (state) + d D (state) c d [C ] [ D] K a b [ A] [ B] (1) Where the terms [A], [B], [C],[D] represent activities, but are frequently approximated as concentrations or partial pressures. (2) Pure liquids (solvent) and solids are not included in an equilibrium constant as they are present in their standard state. (3) The change of temperature has very important effect on the equilibrium constant. (4) The form of reaction equation will have effect on the value of the equilibrium constant. K >>1, indicates a strong thermodynamic tendency to react, so very little of the reactants A and B will remain at equilibrium. K<<1 indicates very little tendency to react and the reverse reaction will be very favorable. INORGANIC CHEMISTRY Section 3 The relationship between Equilibrium constants and rGm r Gm (T ) r Gm (T ) RT ln J J ( Pi i ) (气体反应体系) P A3 orTHE NUCLEAR ATOM Ci i ) (溶液反应体系 An atom consists) of a very small positively charged nucleus, Electron and Nuclei C surrounded by negative electrons held by electrostatic attraction. The motion of electrons changes when chemical If at balanced state, △rG m= 0.are formed, nuclei being unaltered. bonds △rG0m= -RT ln J = —RT ln K = △rH0m-T △rS0m Nuclei)contain positive protons and uncharged neutrons. J = K (structure equilibrium constant Nuclear Theofnumber of is protons thethe atomic number (Z)free of an The equilibrium constant reaction relatedis to standard Gibbs element. The attractive interaction between energy change. Equilibrium constants change strong with temperature in a wayprotons that depends on △H forand the neutrons reaction. is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. J ( INORGANIC CHEMISTRY Section B3 Le Chatelier’ s principle (勒沙特列原理, 吕 查德克原理): It would be applied to qualitatively judge the direction of chemical reaction. a A (state) + b B (state) c C (state) + d D (state) + H Effect factor to the equilibrium: (1) Change of Concentration use the equilibrium constant (2) Change of Pressure use the equilibrium constant (3) The temperature( H ) K :increase with rise in the temperature for an endothermic reaction, and decrease for an exothermic one. INORGANIC CHEMISTRY Section B3 Reaction rates (1) Average velocity It is defined as the increment of the products or decrement of reactant in unitNUCLEAR time. A3 THE ATOM n B r t n r t An atom consists of a very small B positively charged nucleus, surrounded by negative electrons held by electrostatic attraction. The motion of electrons B changes when chemical bonds are formed, nuclei being unaltered. B--the coefficient of B in protons reactionand equation. Nuclei contain positive uncharged neutrons. Nuclear structure The number of protons is the atomic number (Z) of an (a) concentration: element. The attractive strong interaction between protons (b) temperature: Reaction generally increase rise in temperature andrates neutrons is opposed bywith electrostatic repulsion between (c) Catalysis provide alternative reaction pathways of lower energy. Aand truethere catalyst protons. Repulsion dominates as Z increases is by definition can be removed after the And so does not alter the only unchanged a limited number of reaction. stable elements. thermodynamics or the position of equilibrium. Electron and Nuclei INORGANIC CHEMISTRY Section B3 (2) Rare equation: the relationship between the rate and the concentration of the reactant. a A + b B products A3 n [B]m THE NUCLEAR ATOM Rate= k[A] rate constant: k consists of amechanism very small and positively charged nucleus, Electron and Nuclei Orders of reaction: An m, atom n. depend on the not necessarily surrounded by negative equal to the stoichiometric coefficients of theelectrons reactants.held by electrostatic attraction. The motion of electrons changes when chemical For example: nuclei being unaltered. (1) H2+ I2 ==2HI bonds r=k are c(Hformed, 2) c(I2) 1 (2) H2+ Br2 ==2HBr Nuclei contain positive protons k c( Hand )c 2uncharged ( Br ) neutrons. Nuclear structure r 1 2 2 The number of protons is the atomic number k2c( HBr ) (Z) of an 1 (3) H2+ Cl2 ==2HCl element. The attractive strong interaction c( Br2 ) between protons and neutrons is opposed by electrostatic repulsion between 1 protons. Repulsion dominates as Z increases and there is 2 r kconly ( Ha2 limited )c (Cl ) 2 number of stable elements. INORGANIC CHEMISTRY Section B3 a A + b B products Rate= k[A]n[B]m orders of reaction: n = a, m=b. (很少的例子) ——————(基元反应,the process need only one-step reaction from the reactants to products) for most chemical reactions, n a, m b, because the process would be divided into many steps.—————— depend on the mechanism. n + m == the total orders of a reaction The order of reactions ( n + m) The rate equation The unit of rate constant 0 (zero order reaction) Rate = k mol dm-3 s-1 1 (first order reaction) Rate = k c s-1 2 (second order reaction Rate = k c2 mol-1 dm3 s-1 Rate = k c3 mol-2 dm3 s-1 3 (third order reaction) Another means: by designing a lots of favorable experiments. INORGANIC CHEMISTRY Section B3 2 NOg 2H 2 g N 2 g 2H 2 Og 1073k 1 1 1 -1 /( mol L s ) c H /( mol L ) c NO /( mol L ) 试验编号 2 1 2 3 4 5 0.0060 0.0060 0.0060 0.0030 0.0015 Rate = k [c(NO)]n [c(H2)]m n=2 , m = 1. k= ? 0.0010 0.0020 0.0040 0.0040 0.0040 7.9 10 7 3.2 10 6 1.3 10 5 6 6.4 10 3.2 10 6 INORGANIC CHEMISTRY Section B3 (3) Arrhenius equation——the relationship between the rate constant and the temperature. k Ae Ea RT K------the rate constant. Ea-------the activation energy (J.mol-1) Ea ln k ln A RT T-temperature (K) A-------the constant Ea lg k lg A 2.303 R T lnk (or lgk)---1/T : linear relationship the intercept(截距): lnA the slope(斜率): -Ea / R INORGANIC CHEMISTRY Section B3 Ea ln k ln A RT (1) If these data T1,T2, and k1, k2 were given, Ea (T1 T2 ) k1 Ea 1 1 ln ( ) k2 R T2 T1 R T2T1 (2) If many T and k were obtained in the experiment, the method of diagram would be prior to use to calculate the Ea and A. INORGANIC CHEMISTRY Section B3 Problem 3.: Calculate the activity energy of the reaction N2O5 (g)2 NO2 (g)+ 1/2 O2(g) from the relationship (listed in Table) between the temperature and the rate constant. T/K 338 382 318 308 298 273 k/ s-1 4.8710-3 1.5010-3 4.9810-4 1.3510-4 3.4610-5 7.8710-7 Solution 3.: Applying the Arrhenius equation ( k = A e–Ea/RT), lg k = -Ea / (2.303 RT) + lg A. A linear relationship was shown between lg k and 1/T. So, the slope of this line is –Ea / (2.303 R). The data of 1/T and (lg k/s-1) are given in table III T-1/10-3K-1 2.96 3.05 3.14 3.25 3.36 3.66 Lg(k/ s-1) -2.31 -2.82 -3.30 -3.87 -4.46 -6.10 The slope = 2.50 (5.73) 3 5 . 38 10 K 3 3 3.0 10 3.6 10 Ea = 5.38 103 2.303 8.314 = 103.0 103 Jmol-1 = 103 kJmol-1 INORGANIC CHEMISTRY Section B4 B4 OXIDATION AND REDUCTION Definitions (1) Oxidation means combination. with a more electronegative element or the removal of electrons. Reduction means combination with a less electronegative element or the addition of electrons. A complete redox reaction involves both processes. 2H+ (aq)+ Zn (s) Zn2+(aq) + H2(g) (2) A strong oxidizing agent is substance capable of oxidizing many others, and is thus itself easily reduced; A A strong reducing agent is substance capable of reducing many others, and is thus itself easily oxidized. (3) A redox reaction is any reaction involving changes of oxidation state. INORGANIC CHEMISTRY Section B4 Oxidation states Oxidation states of atoms in a compound are calculated by assigning electrons in a bond to the more electronegative element. In simple ionic compounds they are the same as the ionic charges. In any redox reaction the oxidation states of some elements change. Calculation rules of Oxidation states: An element atom consists ofincluded. a very small positively nucleus, (a) Bonds between the same are not Elements havecharged oxidation state 2- theelectrons by O negative zero. In an ion such surrounded as peroxide electronsheld in by theelectrostatic O-O bond are 2 attraction. distributed equally, making O-1. The motion of electrons changes when chemical 2 -formed, nucleielectronegative being unaltered.and electropositive (b) Except in cases suchbonds as Oare the most 2 elements in a compd have an oxidation state equal to the ionic neutrons. charge. Nuclei contain positive protons andnormal uncharged (c) The sum of the oxidation states must equalisthe on the species, The number of protons the charge atomic number (Z) of anand is therefore zero in a neutral compd. thisstrong rule and (b) above, we have H1 in element. The Using attractive interaction between protons H2O, H-1 in CaH2. and neutrons is opposed by electrostatic repulsion between (d) Complex formation, and donor-acceptor interactionas in general don’t alteris the protons. Repulsion dominates Z increases and there oxidation state. only a limited number of stable elements. A redox reaction is any reaction involving changes of oxidation state. INORGANIC CHEMISTRY Section B4 Balancing redox reactions In complete redox reactions the overall changes in oxidation state must A3 THE NUCLEAR ATOM balance. When reactions involve ions in water it is convenient to split the overall reaction into two half reactions. To balance these it may also be An atom consists of a very small positively charged nucleus, Electron and Nuclei necessary to provide water and H+ or OH-. surrounded by negative electrons held by electrostatic attraction. The motion of electrons changes when chemical Half reactions: bonds are formed, nuclei being unaltered. Fe2+ Fe3+ + e Nuclei contain positive protons and uncharged neutrons. (1) Electrons were included in half reaction. Nuclear structure Thebalance numberinof protonsand is the atomic number (Z) of an (2) The half reaction must charges elements. strong interaction between protons MnO4- + 5 e Mn2+element. The attractive (×) 2+ + 4H and is2O opposed MnO4- + 5 e + 8H+ Mnneutrons ( ) by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Section B4 Example 1 Problem 1. Balance the equation H2SO3 + MnO4- SO42- + Mn2+ (in solution). A3acidic THE NUCLEAR ATOM Solution.: (the method of half-reactions) Step 1. Write the two skeletal half-reactions. An atom consists of a very small positively charged nucleus, Electron and Nuclei2H2SO3 SO4 (the reducing agent, H2SO3, is oxidized to SO42-) surrounded by negative electrons held by electrostatic 2+ MnO4 Mn (the oxidizing agent, MnO4-, is reduced to Mn2+); attraction. The motion of electrons changes when chemical Step 2. Balance each half-reaction. bonds are formed, nuclei being unaltered. (i) To balance the O, add water. H2SO3 + H2O SO422+ Nuclei contain positive protons and uncharged neutrons. MnOstructure 4 Mn + 4H2O Nuclear +. 2+ number of protons is the atomic number (Z) of an (ii) To balance the H, The add H H2SO 3 + H2O SO4 + 4 H MnO4- + 8 H+ Mn2+element. + 4H2O The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between (iii) To balance the charge, add electrons. dominates as Z increases and there is H2SO3 + H2O SO42-protons. + 4 H+ +Repulsion 2 ea limited MnO4- + 8 H+ + 5 e-only Mn2+ + 4H2Onumber of stable elements. INORGANIC CHEMISTRY Section B4 Example 1 H2SO3 + H2O SO42- + 4 H+ + 2 e- A3 (× 5) THE NUCLEAR ATOM MnO4- + 8 H+ + 5 e- Mn2+ + 4H2O (× 2) An atom consists of a very small positively charged nucleus, surrounded by negative electrons held by electrostatic Step 3. Add the two half-reactions. Since 2 electrons are lost in the first of electrons changesmultiple when chemical while 5e are gained in attraction. the second,The andmotion since the lowest common of bonds the are formed, unaltered. 2 and 5 is 10, we multiply first by nuclei 5 and being the second by 2. When the Electron and Nuclei resulting equations areNuclei added,contain the 10epositive on the two sidesand of the equations will protons uncharged neutrons. Nuclear structure + cancel. Also 16H and The 5 H2number O will cancel from iseach of protons the side. atomic number (Z) of an The total reaction: element. The attractive strong interaction between protons 22+ + 4 H+ + 3 H O 5 H2SO3 + 2 MnO4- 5 4 + 2Mn 2 andSO neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Example 2 In acidic solution, it is more appropriate to use H+ or H2O. Yet, in alkaline solution, it is more appropriate to use OH- or H2O. (1) Al (s) + 4OH- [Al(OH)4]- + 3e- (2) 2H2O + 2e- 2OH- + H2 (3) 2Al(s)+ 2OH- + 6H2O 2 [Al(OH)4]-+ 3H2 Section B4 INORGANIC CHEMISTRY Section B4 Extraction of the element A3 THE NUCLEAR ATOM Redox reactions are used in the extraction of nearly all elements from natura1ly occurring compounds. Carbon is consists used to of reduce some metal oxides, but many An atom a very small positively charged nucleus, Electron and Nuclei elements require strongersurrounded reducing agents, or theelectrons use of electrolysis. by negative held by electrostatic The methods shown as following common attraction.are Theinmotion of use. electrons changes when chemical (1) carbon bonds are formed, nuclei being unaltered. (2)reduction by using H2 or CO. Nuclei contain positive protonsmetals and uncharged neutrons. (3)the replacement reaction by using the reactive M such as alkali Nuclear structure The number of protons is the atomic number (Z) of an metals or alkaline metals. element. The attractive strong interaction between protons (4)electrolysis and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Section B5 B5 Describing inorganic compounds formulae Stoichiometric (empirical) formulae describe only the relative numbers of atoms present. Molecular formu1ae and/or representations giving structural information should be used when they are appropriate. The physical state of a substance is often specified. INORGANIC CHEMISTRY Section B5 Name Systematic nomenclature can be based on three systems, binary, substitutive (similar to that in organic chemistry) or coordination. Many nonsystematic or trivial(习惯) names are used. A3 THE NUCLEAR ATOM Systematic nomenclature is based on three systems. Binary names: An atom consists of a very small positively charged nucleus, ElectronNaCl: and Nuclei sodium Chloride PCl3: phosphorous trichloride surrounded by negative electrons held by electrostatic N2O4: dinitrogen tetroxide MnO2: Manganese (IV) dioxide attraction. The motion of electrons changes when chemical F-:Fluoride; F2: fluorine. bonds are formed, nuclei being unaltered. Substitutive names: SiH4: silane; SiCl4Nuclei : tetrachloride contain silane positive protons and uncharged neutrons. Nuclear structure NH3: amine; NH2OH: hydroxyamine The number of protons is the atomic number (Z) of an Coordination names: element. The attractive strong interaction between protons [Cu(NH3)4]2+ :tetraaminecopper(2+);tetraaminecopper ion between and neutrons is opposed by electrostatic(II) repulsion [CuCl4]2-:tetrachlorocuprate(2-) or tetrachlorocuprate (II) and there is protons. Repulsion dominates as Z increases [Ni(NH3)6]Br2:hexaaminenickel only a limiteddibromide number of stable elements. K[PF6]: potassium hexafluorophosphate (V) INORGANIC CHEMISTRY A3 Section B5 THE NUCLEAR ATOM Electron and Nuclei Nuclear structure An atom consists of a very small positively charged nucleus, surrounded by negative electrons held by electrostatic attraction. The motion of electrons changes when chemical bonds are formed, nuclei being unaltered. Nuclei contain positive protons and uncharged neutrons. The number of protons is the atomic number (Z) of an element. The attractive strong interaction between protons and neutrons is opposed by electrostatic repulsion between protons. Repulsion dominates as Z increases and there is only a limited number of stable elements. INORGANIC CHEMISTRY Section B5 Structure and bonding A3 THE NUCLEARnumber ATOMand geometry of an atom describe the The coordination number of bonded atoms and their arrangement in space. Oxidation An atom consists a very small positively charged nucleus, states rather than valencies are ofgenerally used for describing Electron and Nuclei by negative electrons held by electrostatic different possiblesurrounded stoichiometries. Bond lengths: attraction. The motion of electrons changes when chemical Bond angles: bonds are formed, nuclei being unaltered. Coordinate number(CN) Nuclear structure INORGANIC CHEMISTRY Section C Section C Structure and bonding in molecules C1 Electron Pair Bonds Lewis and valence Structure A single covalent bond is formed when two atoms share a pair of electrons. Double and triple bonds can be formed when two or three such pairs are shared. —— octet rule A Lewis structure shows the valence electrons in a molecule. Two shared electrons form a single bond, with correspondingly more for multiple bonds. Some atoms may a1so have nonbonding electrons (lone-pairs). Valence structures show the bonds simply as lines. INORGANIC CHEMISTRY :O £º O£º £º H £º H H C H H Section C-1 O: :N N: H Important Notes: Non-bonding electrons or lone-pair electrons localized on one atom would be labeled in Lewis structure. Isoelectronic principle(等电子规律): the molecules or ions having the same number of valence electrons should have similar valence structure. [NH4]+ [BH4]- INORGANIC CHEMISTRY Section C-1 Octets and ‘hypervalence’ In most stable molecules and ions of the elements C-F, each of these atoms has eight electrons (an octet) in its valence shell. Expansion of the octet and increased valency is possible with elements in periods 3 and below. Octet rule: Incomplete octet ———— 缺电子分子 Octet expansion or hypervalence: —— valence expansion or hybrid orbital theory £º F F: £º £º £º F: £º £º B F: :S F F F INORGANIC CHEMISTRY Section C-1 Resonance £º £º 2£- :O£º :O£º £º £º :O £º £º :O£º 2£- :O C :O C £º :O C :O £º :O£º £º 2£- £º £º £º When several alternative valence structures are possible, the bonding may be described in terms of resonance between them. (1) 分散电荷,达稳定结构。 (2) 使所有原子都能满足octet rule. Resonance may also be appropriate with different valence structures that are not equivalent but look equally plausible. For examples: N2O (12) INORGANIC CHEMISTRY Section C-1 Formal Charges: Formal charges are assigned by apportioning bonding electrons equally between the two atoms involved. They can be useful to rationalize apparent anomalies in bonding, and to assess the likely stability of a proposed valence structure. A formal charge on an atom is essentially the charge that would remain if all covalent bonds were broken, with the electrons being assigned equally to the atoms involved. Formal charge = (No. of valence electrons in neutral atom)- (No. of £º £º £º :O£º :O£º £º £º :O £º £º :O£º 2£- :O C :O C £º :O C :O £º :O£º 2£- £º £º £º nonbonding electrons) -1/2 (No. of electrons in bonds formed.) 2£- INORGANIC CHEMISTRY Section C-1 Some general principles for formal charges are: (1) Structure without formal charges are preferred if possible; (2) Structures with formal charges outside the range -1 to +1 are generally unfavorable; (3) Negative formal charges should preferably be assigned to more electronegative atoms, positive charges to more electropositive atoms. For example (P44): CO N2O BF Formal charge is very different from Oxidation state, which is assigned by apportioning electrons in a bond to the more electronegative atom rather than equally. Both are artificial assignments, useful in their respective ways, but neither is intended as a realistic judgment of the charges on atoms. INORGANIC CHEMISTRY Section C-1 Limitations Many covalent molecules and ions cannot be understood in terms of electron pair bonds between two atoms. They include electrondeficient boron hydrides and transition metal compounds. Two-center bonds: localized bonds Three-center bonds: delocalized bonds Molecular Orbital theory: (MOT) INORGANIC CHEMISTRY Section C-1 hybrid orbital theory——解释多原子分子的方向性问题 For example: CH4: Lewis structure: planar real structure: tetrahedron bond angles: 90, real: 10928 Pauling: hybrid orbital theory: 2s 2p ground state of C atom excited state 4 sp3 hybrid orbitals Atom orbitals the foramtion of sp3 hybird orbitals sp3 hybrid orbitals; sp2 ; sp; sp3d (dsp3), sp3d2(d2sp3), dsp2 意义:(1) 解释了多原子分子结构的方向性问题; (2) 中心原子的成键能力增加; INORGANIC CHEMISTRY Section C-1 Types sp sp2 sp3 dsp2 sp3d dsp3 sp3d2 d2sp3 No. of atom orbitals hybridized 2 3 4 4 5 6 No. of hybrid orbitals 2 3 4 4 5 6 The angle between the hybrid orbitals () 180 120 109.5 90 180 120 90 180 90 180 Configuration Linear Trigonal planar Tetrahedral Planar square Trigonal bipyrimdal Octahedral Examples BeCl2 CO2 BF3 NO3- CO32- CH4 CCl4 CHCl3 PdCl42- Complex PCl5 SF6 SiF62- 等性杂化: CH4 (sp3) 不等性杂化: (H2O) (sp3) INORGANIC CHEMISTRY C2 Section C-2 Molecular shapes: VSEPR VSEPR principles The valence shell electron pair repulsion (VSEPR) model is based on the observation that the geometrical arrangement of bonds around an atom is influenced by nonbonding e1ectrons present. It is assumed that electronic pairs – whether bonding or nonbonding - repel each other and adopt a geometrical arrangement that maximizes the distances between them. VSEPR principles have widely applied in predicting the possible steric configuration of covalent molecules for non-transition elements. ————shapes and bond angles of the molecules INORGANIC CHEMISTRY Section C-2 The basic principles of the model are as follows: (i) Valence electron pairs round an atom (whether bonding or nonbonding) adopt a geometry that maximizes the distance between them. The basic geometries usually observed with 2-7 pairs are shown in fig 1. 2. linear sp 3. trigonal planar sp2 A A 5. trigonal bipyramidal sp3d A A A 4. tetrahedral sp3 A 6. Octahedral 7. pentagonal bipyramidal sp3d2 Basic VESPR geometries with 2-7 electron pairs INORGANIC CHEMISTRY Section C-2 (2) Nonbonding electron pairs are closer to the central atom than bonding pairs and have larger repulsions: in fact the order of interaction is: Nonbonding-nonbonding > nonbonding-bonding > bonding-bonding (3) If double (or triple) bonds are present the four (or six) electrons involved behave as if they were a single pair, although they exert more repulsion than do the two electrons of a single bond. (4) As the terminal atoms become more electronegative relative to the central one, bonding electron pairs are drawn away from the central atom and so repel less. INORGANIC CHEMISTRY Section C-2 Using VESEPR It is first necessary to decide which atoms are bonded together. Drawing a valence structure gives the total number of electron pairs around an atom, sometimes known as its steric number. The basic VSEPR geometry is then used to assign positions for bonding and nonbonding e1ectrons. Step1: Designate the central atom: usually the least electronegative one, which does not apply to hydrides or organic groups. Step2: Calculate the steric number (SN) of the central atom, equal to the number of bonded atoms, plus the number of non-bonding electron pairs. Step 3: Give the possible steric configuration of the molecules. Another process to calculate the SN: the No. of electron pairs in valence shell = the total No. of valence electrons in central atom + the No. of the electrons bonded atoms +/- the charge in the ions e.g. 配位原子:H, X, =1; 中心原子:X = 7 O = 0; 中心原子: O = 6 INORGANIC CHEMISTRY Section C-2 Steric numbers 2-4: Linear species (SN=2):BeH2,HgCl2,CO2,N3+, NO2+ Trigonal planar species: (SN=3):BF3, NO3Tetrahedral species(SN=4): CH4 , NH4+, SiCl4 AX2 with SN=3 or 4, bent: NO2- H2O H2S AX3 with SN =4, pyramidal:NH3, PH3 Steric numbers 5 (AX5):—— trigonal bipyramidal AX4: see-saw with two axial and two equatorial X postions, SF4, XeO2F2 AX3:T-shapes, ClF3 AX2: linear: XeF2 INORGANIC CHEMISTRY Steric numbers 6 (AX6):—— octahedral AX5: square pyramidal structure, XeOF4 AX4:square planar; XeF4 Steric numbers 7 (AX7):—— pentagonal bipyramidal IF7 AX5: pentagonal planar, XeF5- Section C-2 INORGANIC CHEMISTRY Section C-2 Extensions, difficulties and exceptions In spite of a lack of firm theoretical foundation the VSEPR model is widely applicable to molecular geometries and even to some solids. Occasionally it fails to predict the correct structure. (1) H2NOH: N as central atom: pyramidal O: bent (2) N(CH3)3 —— pyramidal N(SiH3)3 —— planar INORGANIC CHEMISTRY C3 Section C-3 Molecular orbitals: Homonuclear diatomics Bonding and antibonding orbitals Molecular Orbitals (MOs) are wavefunctions for electrons in molecules. Molecular Orbitals are formed by linear combination of AOs.---LCAO approximation. LCAO: linear combination of atomic orbitals (原子轨道的线性组合形成分子轨道) Overlapping atomic orbitals can give bonding and antibonding MOs 1= c11 + c2 2—— bonding MO(成键分子轨道) 2= c11 - c2 2 —— antibonding MO (反键分子轨道)……..Fig 1. Nonbonding MO(非键分子轨道): An electron in the bonding MO has lower energy than in the AO of an isolated atom, as it has enhanced probability of being close to both nuclei simultaneously. In an antibonding MO an electron has higher energy, with a reduced probability of being in the internuclear region, and internuclear repulsion being “deshielded”. INORGANIC CHEMISTRY Section C-3 MO diagrams An MO diagram is a representation of the energies of bonding and antibonding MOs formed from atomic orbitals. Electrons are assigned to MOs in accordance with the exclusion principle, leading to the same building-up procedure as for the periodic table of elements. (1) 能量相近原则; (2) 最大重叠原则; (3) 对称性匹配原则; (4) 轨道总数目不改变; (5) 轨道通过线性组合后,轨道的能量发生 分裂,1sg能量升高, 1su能量降低, 分子的总能量降低。 1su (6) 电子填充进入分子轨道的原子与电子在 AO中填充的规则相同。 H 1s AO H 1s AO (a) Exclusion principle; (b) Lowest energy principle 1sg (c) Hund’s first rule INORGANIC CHEMISTRY Section C-3 The types of forming MOs (重叠方式来分) 沿键轴方向 ———— 键(head-to-head,头碰头形式): the overlapping of s orbitals or p orbitals or overlapping of s orbital and p orbital. 垂直于键轴方向 —— 键(shoulder-to-shoulder,肩并肩形式): the overlapping of p orbitals. the overlapping of p orbital and d orbitals. Very important Notes: (1)相同符号的原子轨道才能重叠; (2) 两个原子间只有一个键,其他成键均为键。 INORGANIC CHEMISTRY Section C-3 The definition of Bond Order (BO) in MO theory recognizes that a normal single bond is formed by two electrons. BO = (1/2) [(No. of electrons in bonding MOs) - (No. of electrons in bonding MOs)] Fractional values are possible, as in the molecular ions H2+ or He2+, which are both bonded but more weakly than H2. H2+ (1sg)1 BO = (1-0)/2 = 0.5 H2 (1sg)2 BO = (2-0)/2 = 1 He2+ (1sg)2(1su)1 BO = (2-1)/2 = 0.5 1su or 1s * He2 (1sg)2(1su)2 BO = (2-2)/2 = 0 1s AO 1s AO 1sg or 1s INORGANIC CHEMISTRY Section C-3 Second period diatomics In extending the MO theory to second period elements two additional principles need to be considered. (1) Only valence-shell are shown in MO diagrams as inner shells are too tightly bound to be involved in bonding, and don’t overlap significantly. (2) Both 2s and 2p valence orbitals need to be included. Two fundamental rules in the LCAO MO model are important: (1) The number of MOs formed is equal to the total number of starting AOs. (2) Only AOs of the same symmetry type combine to make MOs. (3) Exclusion principle: Lowest energy principle: Hund’s first rule: INORGANIC CHEMISTRY Section C-3 2p atomic orbitals can give rise to both and MOs, the former overlapping along the molecular axis and the latter perpendicular to it. Multiple bonding in O2 and N2 arises from as well as bonding. bonding < bonding < antibonding < antibonding Trends in bond strengths and lengths follow predicted bond orders. The O2 molecule has two unpaired electrons, a fact not predicted by simple electron-pair bonding models. O O2 O O2 (2sg)2(2su)2(2pg)2(2pu)4(2pg)2 BO = 2 2p 2p N2 (2sg)2(2su)2(2pg)2(2pu)4 BO = 3 2s 2s INORGANIC CHEMISTRY Section C-3 用MO描述第二周期的物种及电子的排布情况:P55 Electron configuration BO D(kJmol-1) R (pm) N2 (2sg)2(2su)2(2pg)2(2pu)4 3 945 110 O2+ (2sg)2(2su)2(2pg)2(2pu)4(2pg)1 2.5 630 112 O2 (2sg)2(2su)2(2pg)2(2pu)4(2pg)2 2 498 121 O2(2sg)2(2su)2(2pg)2(2pu)4(2pg)3 1.5 - 128 F2 (2sg)2(2su)2(2pg)2(2pu)4(2pg)4 1 158 142 Higher predicted BO values correspond to stronger and shorter bonds INORGANIC CHEMISTRY C4 Section C-4 Molecular orbitals: Heteronuclear diatomics Basic principles Orbitals from atoms of different elements overlap to give unsymmetrical MOs, the bonding MO being more concentrated on the more electronegative atom. The greater the electronegativity difference, the greater the degree of localization. The basic principle of forming the MO for the heteronulclear diatomics is similar to that of the homonuclear diatomics. (1)LCAO among the AOs having the approximate energy. (2)The number of MOs formed is equal to the total number of starting AOs. (3)the principles of filling the electrons to the MOs INORGANIC CHEMISTRY Section C-4 HF and BH In HF the F 2s orbital is too low in energy to be involved significantly in bonding, but in BH both 2s and 2p orbitals on B can contribute to MOs with H 1s. This situation is described as sp hybridization, and leads to bonding and nonbonding MOs with a spatial localization similar to that assumed in VSEPR theory. H H HF 3 1s 2p Orbital energies Orbital energies B F 3 1 HB 1 2 2p 2 1s 1 2s 2s 1 INORGANIC CHEMISTRY Section C-4 CO When sp hybridization occurs on both atoms in a diatomic the order of MOs can be hard to predict In CO the highest occupied MO (HOMO) is a nonbonding u orbital resembling a lone-pair on C the lowest unoccupied MO (LUMO) is an antibonding orbital. C CO O 4 Very complicated LUMO: (最低未被占用的分子轨道) the Lowest unoccupied MO Orbital energies HOMO: (最高被占用的分子轨道) the highest occupied MO 2 2p 3 2p 2s 1 2 2s 1 INORGANIC CHEMISTRY C5 Molecular orbitals: polyatomics Localized and delocalized orbitals: Polyatomics: Localized orbitals: Two center Delocalized orbitals: three or more center Directed Valence Hybrid Orbital Theroy VSEPR theory: determine the molecular structure Section C-5 INORGANIC CHEMISTRY Section C-5 Multiple Bonds And/or MO orbitals would be formed by LCAO. H H C C C H H O O O C2H4 CO32- Normal bonding 大键 46 INORGANIC CHEMISTRY Section C-5 Three-center Bonding Three-center bonds are necessary for the description of some molecules. Bridge bonds are of the three-center two-electron type; For example: diborane B——H——B (3c-2e bonds) H H B H H B H H B2H6 Al2(CH3)6 and BeH2 Three-center four-electron bonding provides an explanation of some hypervalent molecules. Hydrogen bonds: F——HF (3c-4e bonds) INORGANIC CHEMISTRY C6 Section C-6 Rings and Clusters(簇合物) Introduction Rings and clusters can be formed by using simple two-center bonding models; in others it is necessary to used delocalized MO models. S S S S S N P N S P S S S S P Aromatic Rings Wade’s Rules P INORGANIC CHEMISTRY C7 Section C-7 Bond Strengths Bond enthalpies Bond energies: Mean bond enthalpies are defined as the enthalpy involved in breaking bonds in molecules. H2O (g) 2H + O They may be determined from the thermo-chemical cycles using Hess’ Law. H2+ H1 2H + ½ O2 H2O H2 O H3 = fH0(H2O) 2 B.E. (O-H) H1+H2 +2 B.E (O-H) = fH0(H2O) INORGANIC CHEMISTRY Section C-7 Major Trends Stronger bonds are generally formed with lighter elements in a group, when multiple bonding is present, and when there is a large electronegativity difference between the two elements. Some important trends are summarized below: (1) Bond energies often become smaller on descending a main group. This is expected as electrons in the overlap regions of a bond are less strongly attracted to larger atoms. (2) Bond energies increase with bond order, although the extent to which B(A=B) is larger than B(A-B) depends greatly on A and B, the largest differences occurring with elements from the set C,N,O。 Strong multiple bonding involving these elements may be attributed to the very efficient overlap of 2p orbitals compared with that of larger orbitals in low orbitals. (3) In compounds ABn, with the same elements but different n values, B(A-B) decreases as n increases. The differences are generally less for larger A, more electronegative B. INORGANIC CHEMISTRY Section C-7 (4) Bonds are stronger between elements with a large electronegativity difference. (5) Single A-B bonds where A and B are both from the set N, O, F are weaker than expected from group comparisons. This is often attributed to a repulsion between nonbonding electrons. (6) Other expectations to rule (1) above occur with A-O and A-X (halogen) bonds, which generally increase in strength between periods 2 and 3. This may be partly due to the increased electronegativity difference when A is period 3, but repulsion between lone-pairs electrons on nonbonded atoms may also play a role. INORGANIC CHEMISTRY C8 Section C-8 Lewis acids and bases Definition and scope A Lewis acid (or acceptor) is any species capable of accepting a pair of electrons. for examples: H+, and metal cations, molecules such as BF3 with incomplete octets, and ones such as SiF4 where octet expansion is possible. A Lewis base (or donor) is any species with a pair of non-bonding electrons available for donation. for examples: the molecules such as NH3, and anions such as F-. The Lewis Acid-base definition The Brnsted Acid-bases defination Brǿnsted bases are also Lewis bases, and H+ is a Lewis acid, but Bronsted acids such as HCl are not Lewis acids. INORGANIC CHEMISTRY Section C-8 Lewis acids and bases may interact to give a donor-acceptor complex. BF3 + (CH3)3N: SiF4 + 2 F- (CH3)3N:BF3 [SiF6]2- (the bond of N-B or Si-F is the dative bond ) covalent bond-------dative bond The formation of solvated ions, complexes in solution and coordination compounds are the examples of this types of interactions. INORGANIC CHEMISTRY Section C-8 Modeles of interaction Interaction between the orbitals in Fig.1 will be strongest when the energy difference between the acceptor LUMO and the donor HOMO is least. In this model the best acceptors will have empty orbitals at low energies, the best donors filled orbitals at high energies. By contrast, the strongest electrostatic interactions with take place between the smallest and most highly charged (positive) acceptor, and (negative) donor atoms. INORGANIC CHEMISTRY Section C-8 Hard-soft classification (HSAB classification) Hard donors interact more strongly with hard acceptors, soft donors with soft acceptors (硬亲硬,软亲软,软和硬的反应不容易发生 ). Hard acids tend to be more electropositive, and harder bases more electronegative. Softer donor and acceptor atoms tend to be larger and more polarizable. INORGANIC CHEMISTRY Hard-soft classification Section C-8 It is found that the relative strength of donors depends on the nature of the acceptor and vice versa. The hard and soft acid-base (HSAB)classification is often used to rationalize some of the differences. When two acids (A1 and A2) and in competition for two bases (B1 and B2) the equilibrium. A1:B1 + A2:B2 Al:B2 + A2:B1 will lie in the direction where the harder of the two acids is in combination with the harder base, and the softer acid with the softer base. As a standard for comparison the prototype hard acid H+ and soft acid [(CH,)Hg]+ are often used. Thus the equilibrium [B:H]+ + [(CH,)Hg]+ H+ + [(CH,)Hg:B]+ will lie to the left or right according to the degree of hardness of the base B. Examples of hard acids are H+, cations of very electropositve metals such as Mg2+, and nonmetal fluorides such as BF3. Soft acids include cations of late transition and post-transition metal such as Cu+, Pd2+ and Hg2+. The hardness of bases increase with the number of the donor atom (e.g. NH3 < H2O < F-) and decreases down any group (e.g. NH3 > PH3, and F- > Cl- > Br- > l- ). INORGANIC CHEMISTRY Section C-8 Hard-soft classification Hard Hard-soft Soft Acids H+, Li+, Na+, Be2+, Mg2+, Ca2+, Cr3+, RE3+, BF3, SO3 Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+, SO2, BBr3 Cu+, Ag+, Ti+, Hg+, Pd2+, Cd2+, Pt2+, Hg2+, BH3 Bases F-, OH-, H2O, NH3, CO32-, NO3-, O2-, SO42-, PO43-, ClO4-, NO2-, SO32-, Br-, N3-, N2, C5H5N, SCN- H-, R-, CN-, CO, I-, NCS-, R3P, R2S, C6H6, S2- Hard-hard interactions have a greater electrostatic component and soft-soft ones depend more on orbitals interactions. But many other factors may be involved. Soft acceptor and donor atoms are often large and Van der Waals’ force may contribute to the bonding; some soft bases such as CO also show - acceptor behavior is defined in a competitive situation. INORGANIC CHEMISTRY Section C-8 Polymerization Formation of dimers and polymeric structure is a manifestation of donor-acceptor interaction between molecules of the same kind. 2 AlCl3 Al2Cl6 Cl Cl Al Al Cl Cl Cl Cl Hydrogen Bonding can also be regarded as a donor-acceptor interaction in which the acceptor LUMO is the unoccupied antibonding orbital of hydrogen bonded to an electronegative element. F—H---F INORGANIC CHEMISTRY C9 Section C-9 Molecules in Condensed Phases Molecular solids and liquids Intremolecular forces cause molecular substances to condense to form solids and liquids. Enthalpies of fusion (melting, 熔解热) and vaporization (汽化热) Molecular solids: molecular retain their identity with geometries similar to those in the gas phase. with highly unsymmetrical the directional nature of intermolecular forces Molecular liquids: more disorganized than molecular solids INORGANIC CHEMISTRY Section C-9 Intermolecular forces With polar molecules, the force between permanent electric dipoles is the dominant one. When polarity is absent, the force arises from the interaction between instantaneous dipoles. ------- the London dispersion or Van der Waals’ force Fig 1. Polarizability generally increases with the sizes atoms, and the sequence of boiling points He< Ne <Ar < Kr. The boiling point increase down the group in most series of nonpolar molecules, for examples, CH4 < SiH4 < GeH4. CF4 < CCl4 < CBr4 < CI4 Hydrogen bonding: (P77, Fig 1) NH3 H2O 沸点、酸性 HF 的某些性质的反常表现。 INORGANIC CHEMISTRY Section C-9 Molecular Polarity Dipole moment (, Debye, 德拜): = q (imagine charge)× d (distance) Polarity is a measure of charge separation in bonds and can often be related to the electronegativtiy difference between the atoms. HF (6.4), HCl (3.6), HBr (2.7), HI (1.4) (1) Lone-pair electrons also have dipole moments. (2) In polyatomic molecules the dipoles associated with each bond add vectorially to give a resultant that depends on the bond angles. The highly symmetrical molecules such as BF3, CF4, PF5, the net dipole moment is zero even though the individual bonds may be strongly polar. (3) Dielectric constant: molecular dipole moments (4) In nonpolar substances the dielectric constant arises from the molecular polarizability, and is generally much smaller than with polar molecules. INORGANIC CHEMISTRY Section D-1 Section D D1 Introduction to solids Crystals and glasses A crystalline solid is characterized by a unit cell containing an arrangement of atoms repeated indefinitely. Long-range order: in crystal For a crystal it is only necessary to give the details of one unit cell. The substances are said to have the same structure if the arrangement of atoms within a unit cell are different. ------X-ray structure analysis method or X-ray diffraction Polymorphism (同质异晶现象): TiO2: rutile (金红石), brookite (板钛矿), anatase (锐钛矿) Non-crystalline or glassy solids do not have a unit cell. Short-range order: local chemical bonding arrangement Short-range order resulting from the local bonding of atoms may, however, be similar in crystals and glasses. Unit cell(晶胞): 晶体的最小重复单元,通过晶胞在空间 平移无隙地堆砌而成晶体 Unit cell parameter(晶胞参数): a,b,c,α,β,γ; Crystal system Cubic trigonal tetragonal hexagonal orthogonal Monoclinic triclinic Unit cell parameter a=b=c a=b=c a = b≠c a = b≠c a≠b≠c a≠b≠c a≠b≠c α=β=γ= 900 α=β=γ≠900 α=β=γ= 900 α=β= 900, γ= 1200 α=β=γ= 900 α=β= 900, γ≠ 900 α≠β≠γ≠ 900 compds NaCl Al2O3 SnO2 AgI HgCl2 KClO3 CuSO4·5H2O INORGANIC CHEMISTRY Section D-1 Looking at unit cells Different representations of unit cel1s are possible. It is important to understand how to use them to determine the stoichiometry of the compound(化学计量比,即组成) and the coordination of each atom(原子坐标). To specify the complete structure of a crystalline solid it is only necessary to show one unit cell, but interpreting these Pictures requires practice. Figure 1 shows some views of the cesium chloride structure (CsCl, depicted as MX). (see below). --P 81, Fig 1. INORGANIC CHEMISTRY 透射图 (斜射投影图) (a) Figure 1a is a perspective view (透视图) (more correctly known as a clinographic projection (斜射投影图), which is the most common way of showing a unit cell. Section D-1 轴向投影图 (b) Figure 1b shows a projection down one axis of the cell (轴向投影图). The Position of an atom on the hidden axis is given by a specifying a fractional coordinate (e.g. 0.5 for the central atom showing it is halfway up). No coordinate is given for atoms at the base of the cell. INORGANIC CHEMISTRY (c) Figure 1c shows the atoms shifted relative to the unit cell, and emphasizes the fact that what is important about a unit cell is its size and shape (晶体尺 寸与形状); its origin is arbitrary because of the way in which it is repeated to fill space. Section D-1 (d) In Figure.1d the drawing has been extended to show some repeated positions of the central atom. This helps in seeing the coordination of the corner atom (给出离子的配位情况). INORGANIC CHEMISTRY Section D-1 Stoichiometry (计量比): the relative numbers of different types of atoms. counting all the atoms depicted, and taking account of those that are shared with neighboring cells. Any atom at a corner of a unit cell is shared between eight cells, any at an edge between four, and any on a face between two. the Coordination of each atom (配位情况): INORGANIC CHEMISTRY Section D-1 Non-stoichiometry (非化学计量比) 非化学计量比化合物, 非整比化合物 Some solids, especially natural minerals and transition metal compounds, are nonstoichiometric with variable composition. 1) Defects (晶格缺陷) Vacanies (空位,atoms missing from their expected sites.) Interstitials (间隙,填隙原子,extra atoms in sites normally vacant in the unit cell) An imbalance of defects involving different elements can introduce nonstoichiometry. for example: sodium tungsten bronzes NaxWO3 (x = 0 ~ 0.9 ) 2) Replacement(同晶置换): aluminosilicate feldspars (Na, Ca) (Al, Si)4O8 INORGANIC CHEMISTRY Section D-1 Chemical Classification Solids are often classified according to their chemical bonding, structures and properties: 1) Molecular solids(分子晶体) contain discrete molecular units held by relatively weak intermolecular forces; ——low melting point, low boiling point, low rigidity, insulator, etc 2) Metallic Solids (金属晶体) have atoms with high coordination numbers, bound by delocalized electrons that give metallic conduction;--thermal and electrical conduction. 3) Covalent or polymeric solids (原子晶体) have atoms bound by directional covalent bonds giving relatively low coordination numbers in a continuous one- or two- three- dimensional network;—— high rigidity, high m.p. and b.p., insulators, etc 4) Ionic Solids (离子晶体) are bound by electrostatic attraction between anions and cations with structures where every anions is surrounded by cations and vice versa. high m.p. and b.p., brittleness, insulator in solid state, etc INORGANIC CHEMISTRY Section D-1 INORGANIC CHEMISTRY Section D-2 D2 Element structures Sphere packing Element structures where chemical bonding is non-directional are best introduced by considering the packing of equal spheres. ———— Close-packed structures (球密堆积形成晶体) Spheres of equal size may be packed in three dimensions to give hexagonal closepacked (hcp) and cubic close-packed (ccp, also known as face-centered cubic, (fcc) structures. The body-centered cubic (bcc) structure is slightly less efficiently close packed. ABABABABABAB….. Hexagongal close packing (hcp, 六方密堆积, 74.05%) ABCABCABCABC……Cubic close packing (ccp, 立方密堆积, 52.36%) body-centered cubic (bcc) structure : (体心立方密堆积,68.02%) INORGANIC CHEMISTRY Section D-2 Simple cubic close packing: (简单立方密堆积) C.N.: 8 2r a Efficient Space filling: 52.36 % 4 3 r 3 100% 52.36% 3 a INORGANIC CHEMISTRY 体心立方堆积:bcc 配位数:8 空间占有率: 68.02% Section D-2 4r 3a 4 2 r 3 3 100% 68.02% 3 a INORGANIC CHEMISTRY Section D-2 b f h b h b b b f f h b b 面心立方密堆积:fcc c 1.633a, b a 第三层与第一层有错位,以ABCABC… 方式排列 空间占有率: 74.05% 4 3 r 3 100% 74.05% abc sin 120 2 INORGANIC CHEMISTRY Section D-2 b f h h f f h b 第三层与第一层对齐,产生ABAB…方式 配位数:12 ; 空间占有率:74.05% b b b 六方密堆积 Hcp b b INORGANIC CHEMISTRY Section D-2 Hexagongal close packing Cubic close packing fcc INORGANIC CHEMISTRY Section D-2 Metallic elements Many metallic elements have hcp, fcc or bcc structures. There are some clear group trends in structure, although there are exceptions to these and some metals have less regular structures, especially in the p block. Some factors: depend on temperature and/or pressure some regularities (the numbers of valence electrons ) The commonest stable structures according to group numbers are: Group 1 ( IA):bcc Group 2 (IIA): varied Group 3,4 (IIIB, IVB): hcp Group 5, 6 (VB, VIB): ccp Group 7, 8 (VIIB, VIII): hcp Group 9-11 (VIII, IB): fcc INORGANIC CHEMISTRY Section D-2 Nonmetallic elements Single-bonded structures where each element achieves an octet lead to the following predictions. Group 14 (IVA):four tetrahedral bonds as shown in the diamond structure of C, Si, Ge, and Sn. Group 15 (VA): three bonds in a pyramidal (non planar ) geometry, which can give rise to P4 molecules (white phosphorus) or a variety of polymeric structures shown by P and As. Phosphorus has several allotropes, some with apparently complex structures, but all are based on the same local bonding. Group 16 (VIA): Two bonds, noncolinear, as found in S8 rings and in spiral chains with Se and Te. The different allotropes of sulfur all have this bonding. Group 17 (VIIA): one bonds, giving diatomic molecular structures shown by all the halogens. Group 18(0 group): no bonds, leading to monatomic structures with atoms held only by van der Waals’ forces. The normal solid structure of the noble gas elements is fcc. INORGANIC CHEMISTRY Section D-2 The structural chemistry of the period 2 elements C, N, and O shows a greater tendency to multiple bonding than in lower periods. Carbon: graphite, diamond, fullerence nitrogen: N N, oxygen: O =O INORGANIC CHEMISTRY Section D-3 D3 Binary compounds: simple structures Coordination number and geometry Binary compounds: ones with two elements present Simple crystal structures: ones in which each atom (or ions) is surrounded in a regularly way by atoms (or ions ) of the other kind. Coordination number(配位数): In regularity binary solids, the ratio of CN values must reflect the stiochiometry. For example: in AB, both A and B have the same CN. zinc blende(4:4); rocksalt (6 : 6); in AB2, the CN of A must be twice that of B. Rutile (6:3), CdI2, (6:3) INORGANIC CHEMISTRY Section D-3 Coordination geometry (配位构型): In the structures shown many of the atoms have a regular coordination geometry. CN = 2, linear (B in ReO3 ); CN = 3, planar (B in rutile (金红石)); CN=4,tetrahedral (A and B in zinc blende(闪锌矿), B in fluorite(萤石); CN=6,octahedral (A and B in rocksalt (岩盐矿),A in NiAs, rutile, and CdI2); CN= 8, cubic (A and B in CsCl, A in fluorite) Other geometries are sometimes found, especially for the nonmetal B atom: CN =2, bent, (SiO2 structures); CN=3, pyramidal (in CdI2); CN=6, trigonal prismatic (in NiAs) INORGANIC CHEMISTRY Section D-4 D4 Binary compounds: Factors influences structures Ionic Radii Ionic radii(离子半径) are derived from a somewhat arbitrary division of the observed anion-cation distances. Sets of ionic radii are all based ultimately on somewhat arbitrary assumptions. Four kinds of ionic radius: (a) Goldschmidt ionic radius: standard: O2-: 132pm. F-: 133 pm (b) Pauling ionic radius: standard: O2-: 140pm. F-: 136 pm (c) Shannon and Prewitt ionic radius, based on the assumed radius of 140 pm for O2- in six coordination. (有效离子半径) (d) Yatsmirskii ionic radius: (热化学离子半径) Any consistent set of radii should be able to give estimates of the total anion-cation distance and hence the unit cell dimensions if the structures is known. It is essential not to mix values from different sets. Although the values may differ, all sets show the same trends. INORGANIC CHEMISTRY Section D-4 Table Ionic radii (pm) for six coordination, based on a value of 140 pm for O2Li+ Be2+ 27 Na+ 102 Mg2+ 72 Al3+ 53 K+ 138 Ca2+ 100 Ga3+ 62 Rb+ 149 Sr2+ 116 Cs+ Ba2+ 149 76 167 H- 146 O2- 140 F- 133 S2- 182 Cl- 167 Br- 182 I- 206 (i) For isoelectronic ions, radii decrease with increasing positive charge (Na+ > Mg2+ > Al3+) or decreasing negative charge. (ii) Radii increase down each group; (iii) For elements with variable oxidation state, radius decreases with increasing positive charge; (iv) Most anions are larges than most cations. (v) Ionic radii increase with coordination number (CN). For example, the Shannon and Prewitt radii for K+ with different CN are 138(6), 151 (8), 159 (10), 160 (12). INORGANIC CHEMISTRY Section D-4 Radius ratios An ionic solid should achieve maximum electrostatic stability, when (i) each ion is surrounded by as many as possible ions of opposites charges; (ii) the anioncation distance is as short as possible. The radius ratio rules(半径比规则): predict the likely CN for the smaller ion (usually the cation) in terms of the ration r>/r< where r< is the smaller and r> the larger of the two radii, the approximate radius ratios for different CN are: r>/r< > 0.7 CN 8 0.4 ~ 0.7 6 For examples: BeF2: 0.20 CN=4; MgF2: 0.54 6 CaF2 : 0.75 8 0.2~ 0.4 4 INORGANIC CHEMISTRY Section D-4 Ion Polarizability Polarizabilty of the ion: refers to the ability of an applied electric field to distort the electron cloud and so induce an electric dipole moment. The most polarizable ions are large ones, especially anions from later periods (S2-, I-, Br-). In layer and chain structure, the anions are generally in asymmetric environments and experiences a strong net electric field from neighboring ions. Polarization lowers the energy of an ion in this situation , giving a stabilizing effect not possible when the coordination is symmerical. Layer structures occur frequently with disulfides and dichlorides (and with heavier anions lower in the same groups), but almost never with difluorides and dioxides: TiO2 and FeF2; TiS2 and FeI2. INORGANIC CHEMISTRY Section D-4 Covalent bonding Purely electronstatics forces between ions are nondirectional, but with increasing covalent character the directional properties of valence orbitals become more important. Compounds between nonmetallic elements have predominantly covalent bonding and the structures can often be rationalized from the expected CN and bonding geometry of the atom presents. INORGANIC CHEMISTRY Section D-6 D6 Lattice energies The Born-Haber Cycle The lattice energy UL of a solid compound is defined as the energies required to transform it into gas-phase ions: (1) NaCl (s) Na+ (g) + Cl- (g) UL (positive value) (2) Na+ (g) + Cl- (g) NaCl (s) The reverse process ------- UL (negative value) Lattice energies may be estimated from a thermodynamic cycle knows as a Born-Haber cycle, which makes uses of Hess’ Law. INORGANIC CHEMISTRY Section D-6 Lattice enthalpy HL= -fH (NaCl) + atH(Na) + ½ B(Cl2) + I (Na) – A (Cl) the enthalpy of formation of NaCl: fH (NaCl) the enthalpy of atomization of Na solid: atH (Na) the bond enthalpy of Cl2: B(Cl2) the ionization energy of Na: I (Na) the electron affinity of Cl: A (Cl) INORGANIC CHEMISTRY Section D-6 1) Thermodynamic data —— Hess’ Law 2) The other methods for lattice energy (theoretical estimates): (a) Born- Mayer equation The long-range Coulomb interaction between charges. N 0 Az z e 2 1 UL (1 ) 40 r0 n (b) Kapustinskii equations: C z z UL r r (1) lattice energies increase strongly with increasing charge on the ions. (2) lattice energies are always larger for smaller ions. INORGANIC CHEMISTRY NaCl 型 离子晶体 NaF NaCl NaBr NaI MgO CaO SrO BaO Section D-6 Z1 Z2 r+ /pm 1 1 1 1 2 2 2 2 1 1 1 1 2 2 2 2 95 95 95 95 65 99 113 135 rU /pm /kJ·mol-1 136 920 181 770 195 733 216 683 140 4147 140 3557 140 3360 140 3091 熔点 /o C 992 801 747 662 2800 2576 2430 1923 硬度 3.2 2.5 <2.5 <2.5 5.5 4.5 3.5 3.3 INORGANIC CHEMISTRY Application Section D-6 INORGANIC CHEMISTRY Section D-6 (ii) Stabilization of high and low oxidation states When an element has variable oxidation states, it is often found that the highest value is obtained with oxide and/or fluoride. The ionic model again suggests that a balance between IE and lattice energy is important. Small and/or highly charged ions provide the highest lattice energies according to equation 1. and increase in lattice energy with higher oxidation state is more likely to compensate for the high IE. A large ion with low charge such as I- is more likely stabilize a low oxidation state, as the smaller lattice energy may no longer compensate for high IE input. CuF2 CuCl2, CuBr2() CuF (×) or CuX (X=Cl, Br I) INORGANIC CHEMISTRY Section D-6 (III) Stabilization of large anions or cations (a) Large cations stabilize large anions. (b) Oxoanions salts such as carbonates are harder to decompose thermally when combined with large cations. (c) Solids where both ions are larger are generally less soluble in water ones with a large ion and a small one. A satisfactory explanation depends on a balance of energies. INORGANIC CHEMISTRY Section D-7 D7 Electrical and optical properties of solids The band model (能带模型) The band model: an extension of the molecular orbital method The overlap of atomic orbitals in an extended solid gives rise to continuous bands of electronic energy levels associated with different degrees of bonding. In a simple monatomic solid the bottom of the band is made up of orbitals bonding between all neighboring atoms; orbitals at the top of the band are antibonding, and levels in the middle have an intermediate bonding character. Metallic and nonmetallic behavior results from a band partially occupied by electrons, so that there is no energy gap between the top filled level and the lowest empty one. On the other hand, a nonmetallic solid has bandgap between a completely filled band (the valence band) and a completely empty one (the conduction band) So, different atomic orbitals can, in principle, give rise to different bands. INORGANIC CHEMISTRY the distinction between nonmetallic and metallic solids. Band gap(带隙,能隙): Valence band (价带): a completely filled band Conduction band (导带): a completely empty one Section D-7 INORGANIC CHEMISTRY Section D-7 Nonmetallic solids include ionic and covalent compounds. In the former case, the VB is made up of the top filled anion levels and the CB of the lowest empty cation levels. In covalent solids, such as diamond the VB consists of bonding orbitals and the CB of antibonding orbitals. Simple metallic solids are elements or alloys with close-packed structures where the large number of interatomic overlaps gives rise to wide bands with no gaps between levels from different atomic orbitals. INORGANIC CHEMISTRY Section D-7 Band gaps Band edges determine the optical absorption of nonmetallic solid and the possibility of semiconduction. Band gaps in binary solids decrease with decreasing electronegativity difference between the elements. In both ionic and covalent solids bandgaps are smaller with elements in lower periods. A solid with a small bandgap is a semiconductor with a conductivity that (unlike the case with a metal) increases as temperature is raised. The bandgap also determines the minimum photo energy required to excite an electron from the VB to CB, and hence the thresold for optical absorption by a solid. In covalent solid the bandgap is related to the energy splitting between bonding and antibonding ortibals. And thus to the strength of bonding, In an ionic solid the bandgap is determined by the energy required to transfer an electron back from the anion to cation, which is related to the lattice energy. Some systematic trends: (a) In a series of isoelectronic solids, such as CuBr-ZnSe-GaAs-Ge the bandgap decrease with decreasing electronegativity difference between the two elements. (b) In series such as C-Si-Ge- or LiF-NaF-KF the bandgap decreases as the group is descended and atoms or ions become larger. INORGANIC CHEMISTRY Section D-7 Dielectric properties The dielectric constant of a medium is a measure of the electrostatic polarization, which reduces the forces between charges. the static dielectric constant: depend on the displacement of ions from their regular positions in an applied electric field. It is applicable for static fields, or frequencies of electromagnetic radiation up into the microwave range. The high-frequency dielectric constant: is measured at frequencies faster than the vibrational motion of ions. ----applicable in the visible region of the spectrum and determines the refractive index(折射率). ——depend on electronic polarizability. Large ions contribute to this: Glass containing Pb2+used for lenses——a high refractive index. TiO2(钛白粉): INORGANIC CHEMISTRY Section D-7 Influence of defects Defects including impurities have a major influence on the electrical properties of nonmetallic solids. They can provide extra electrons or holes, which enhance semiconduction, and they can also facilitate conduction by ions. All solids contain defects where the regularity of the ideal periodic lattice is broken. Line and plane defects: for mechanical properties; Point defects: for electrical properties; They include (a) Vacancies or atoms missing from regular lattice positions; (b) Interstitials or atoms in positions not normally occupied; (c) Impurities either accidentally present or introduced as deliberate doping. Defects that introduce extra electrons, or that give missing electrons or “holes”, have a large influence on electronic conduction in nonmetallic solids. INORGANIC CHEMISTRY Section D-7 Providing the electrons: N-type semiconductor: doping Si with P replaces some tetrahedral bonded Si atoms in the diamond lattice with P. Each replacement provides one extra valence electron, which requires only a small energy to escape into the CB of silicon. P-type semiconductor: replacing an Si atom with Al gives a missing electron of hole. Other examples: the slight reduction pf TiO2: add some electrons---------a n-type semiconductor. Oxidation of NiO: decrease some electrons-------a p-type semiconductor Ionic conduction: atoms in defect sites may themselves be mobile these would be correlated with the number of defects, and may be especially large in disordered solids. INORGANIC CHEMISTRY Section E1 Section E E1 Solvent types and properties Polarity and solvation A solvent is a liquid medium in which dissolved substances are known as solutes. application: (a) storing substances that would otherwise be in inconvenient states (gases, eg. NH3). (b) facilitating reactions that would otherwise be hard to carry out (solids and liquids). Important: controlling what substances dissolve easily and what types of reactions may be performed. The chemical as well as the physical state of solutes may be altered by interaction with the solvent. INORGANIC CHEMISTRY Section E1 Polarity: polar molecular. (1) The molecules with large dipole moments form polar solvents, such as NH3, H2O, etc; dielectric constant (r): solvation (溶剂化): involves donor-acceptor interactions solvent molecules are ordered round the solute, not only in the primary solvation sphere but affecting more distant molecules. (2) That with little or no dipole moments form nonpolar solvents, such as CO2, hexane, benzene, etc. they are better at dissolving mompolar molecules and for carrying out reactions where no ions are involved. Between nonpolar solvent and solute: Van der Waals’ force Nonpolar solvent are generally poor solvents for polar molecules. Nonpolar molecules cannot compete with the strong intermolecular forces in a polar solvent. —— 相似相溶原理 INORGANIC CHEMISTRY Section E1 INORGANIC CHEMISTRY Section E1 Donor and acceptor properties (1) Donor or Lewis Base: most polar solvents : lone-pair electrons H2O, NH3, C5H5N (Py), etc. solvating cations and other Lewis acids.: (2) Acceptor or Lewis Acids: empty of from hydrogen bonding solvating anions. (3) Solvolysis (溶剂解反应): OPCl3 + 6 H2O OP(OH)3 + 3 H3O++ 3 Cl- OPCl3 + 6 NH3 OP(OH)3 + 3 NH4++ 3 Cl- FeCl3 in different solvents: for the difference of polarity and the solvation of small ions. FeCl3 + pyridine (C5H5N) [FeCl3Py] FeCl3 + DMSO {(CH3)2SO} [FeCl2DMSO4]+ + Cl- FeCl3 + MeCN [FeCl2MeCN4]+ + [FeCl4]- INORGANIC CHEMISTRY Section E1 Ion-Transfer solvents (1) Protic solvents (质子溶剂): have transferable H+ water, NH3,C2H5OH,CH3OH, etc Autoprotolysis (自质子解): 2 H2O H3O + + OH2 NH3 NH4+ + NH2An acid is the positive species (NH4+ ) formed or any solute that gives rise to it. A base is the negative species (NH2-) formed or anything producing it in solution. (2) Aprotic Solvents (非质子溶剂):don’t have transferable H+, but some other ion such as halide or oxide can be involved. autoionization: ion transfer 2 BrF3 + SnF4 2 BrF2+ + SnF62BrF3 --------- Lewis base to produce F- ions. SnF4---------- Lewis acid to react with F- ions INORGANIC CHEMISTRY (3) Lux-Flood acid/base definition in oxide melts the solvent system. An oxide donor-------- a base ; An oxide acceptor -----an acid CaO (base) + SiO2 (acid) CaSiO3 Section E1 INORGANIC CHEMISTRY E2 Section E2 Brønsted Acids and Bases Definitions Brønsted acids: a proton donor ; Brønsted bases: a proton acceptor an acid-base reaction involves protolysis HA + B HB+ + A- Conjugate Acid- Base relationship(共扼酸碱关系): Conjugate Acid- Base pair (共扼酸碱对): For example: HCl / Cl- , H2O/OH-, H3O+ / H2O, NH4+/NH3 H2PO4-/HPO42- HPO42- / PO43- H2SO4/HSO4- This definition of acids and bases should not be confused with Lewis acid-base definition. Brønsted bases are also Lewis bases. H+ _____ Lewis acid and Brønsted acid, BF3 _____ Lewis acid, not Brønsted acid Brønsted Acidity is solvent dependent. Substances such as HCl are covalent molecules that undergo protolysis only in solvents polar enough to solvate ions and when a base is present. INORGANIC CHEMISTRY 酸 HAc H 2 PO HPO NH 4 2 4 4 [CH 3 NH 3 ] [Fe(H 2 O) 6] [Fe(OH)(H 2 O) 5 ] 3 2 Section E2 H+ +碱 H Ac 2 H HPO 4 3 H PO 4 H NH 3 H CH 3 NH 2 H [Fe(OH)(H 2 O) 5 ] 2 H [Fe(OH) 2 (H 2 O) 4 ] INORGANIC CHEMISTRY Section E2 c(H3O ) c(OH ) KW c c pH value: Water undergoes autoprotolysis (self-ionization): 2 H2O H3O + + OHThe equilibrium constant: Kw = [H3O+][OH-] = 1×10-14 (25℃) T , KW In pure water and in solutions that don’t provide any additional source of H3O+ or OH- both ions have molar concentrations equal to 10-7. pH scale: pH = -lg [H+] At 25 ℃, Neutral water: pH = 7 pH < 7 , acidic solution [H+] > [OH-] pH > 7 , basic solution (alkaline) [H+] < [OH-] INORGANIC CHEMISTRY Section E2 Strong and weak behavior HA + H2O H3O + + AAcidity constant or acid dissociation constant of HA: pKa = -lg Ka [ H 3O ][ A ] Ka [ HA] a larger Ka value corresponds to a smaller pKa. pKa < 0, strong acid, the equilibrium lies strongly to the right, H2SO4, HCl, etc. pKa > 0, weak acid, and undergo only partial protolysis. HF, HSO4B + H2O OH- + HB+ basicity constant pKb = -lg Kb = 14- pKa Strong base: pKb < 0 Weak base: pKb > 0 [ HB ][OH ] Kb [ B] INORGANIC CHEMISTRY Section E2 INORGANIC CHEMISTRY Section E2 the degree of ionization: (电离度) Degree of electrolytic dissociation: HA + H2O H3O + + A- (for weak monoatomic acid (一元弱酸)) C1 (initial state) C2 (the balanced state) = C2/C1 × 100% [H3O+] = C1 × [ H 3O ][ A ] [ H 3O ]2 c 2 Ka [ HA] c [ H 3O ] 1 (1) If < 5%, or c/Ka > 500; pH = - lg [H+] (2) If > 5%, K a c 2 [ H 3 O ] c K a c 2 K K a a 4K ac [H 3 O ] 2 INORGANIC CHEMISTRY Section E2 For weak monoatomic base: B +H2O OH- + BH+ [ HB ][OH ] [OH ]2 c 2 Kb [ B] c [OH ] 1 (1) If < 5%, or c/Ka > 500; (2) If > 5%, 2 K K b b 4Kbc [OH ] 2 pOH = - lg [OH-] pH = 14- pOH [OH ] K b c INORGANIC CHEMISTRY Section E2 Common ion effect(同离子效应): The degree of ionization would decrease for being the common ions in solution. HAc(aq) + H2O (l) H3O+ (aq) + Ac–(aq) NH4Ac(aq) NH (aq) + Ac–(aq) 4 If addition of HCl, the same phenomenon would be observed. INORGANIC CHEMISTRY Section E2 For example: Calculate the pH value and the degree ionization of the mixture of HCl (0.10 mol·L-1) and HAc (0.10mol·L-1). (Ka = 1.75×10-5) If no HCl, y = 1.33 ×10-3 mol·L-1 ,’ = 1.33×10-3 / 0.10 ×100 % = 1.33 % pH1 = 2.876 HAc + H2O H3O+ + Ac- Initial state(mol·L-1) 0.10 Balanced (mol·L-1) 0.10 – x x + 0.1 x [ H 3O ][ Ac ] x (0.10 x) Ka [ HAc] 0.10 x x << 0.10 , x = Ka = 1.75×10-5 mol·L-1 pH = 1.000 = 1.75×10-5 / 0.10 ×100 % = 0.018 % 例:在 0.10 mol·L-1 的HAc 溶液中,加入 NH4Ac (s),使 NH4Ac的浓度为 0.10 mol·L-1,计 算该溶液的 pH值和 HAc的解离度。 解: HAc(aq)+H2O(l) 0.10 ceq / (mol·L-1) 0.10 – x c0/ (mol·L-1) x (0.10 x) 5 1.8 10 0.10 x H3O (aq)+Ac-(aq) + 0 x 0.10 0.10 + x 0.10 ± x ≈ 0.10 x = 1.8×10-5 c(H+) = 1.8×10-5 mol·L-1 pH = 4.74,α = 0.018% 0.10 mol·L-1 HAc溶液:pH = 2.89,α = 1.3% INORGANIC CHEMISTRY Section E2 Salt effect The degree of ionization () of weak electrolyte would increase if adding some kind of salt into the solution. Owing to increasing of electrostatic attraction. Common ion effect: For weak polyatomic acid or base, fractional ionization H 3O (aq) HCO- 3 (aq) 第一步:H 2 CO3 (aq) H 2 O(l) c(H O )c(HCO ) (H CO ) 4.2 10 K a1 3 2 c(H 2CO3 ) 3 第二步:HCO (aq) H 2 O(l) H 3 O (aq) CO - 3 K a1 K a2 2- 3 3 2 3 7 2- 3 c(H O )c(CO ) (H CO ) 4.7 10 c(HCO ) K a2 - 3 (aq) 11 - 3 10 3 溶液中的H 3 O 主要来自于第一步解离反应, c(H 3 O )的计算可按一元弱酸的解离平衡 做近似处理。 INORGANIC CHEMISTRY Section E2 For weak polyatomic acid or base, fractional ionization H2S + H2O HS- + H3O+ Ka1 = 5.7 ×10-8 HS- + H2O S2- + H3O+ Ka2 = 1.5×10-15 例题:计算 0.010 mol·L-1 H2CO3溶液中的 H3O+, H2CO3, HCO-3,CO32-和OH-的浓度以及溶液的pH值。 H 2 CO 3 (aq) H 2 O(l) 解: 1 H 3 O (aq) HCO- 3 (aq) 0.010 -x 平衡浓度 /( mol L ) x K a1 (H 2 CO 3 ) 4.2 10 x 7 c(H O )c(HCO ) - 3 3 c(H 2 CO3 ) 0.010 - x 0.010 2 x 0.010-x x 6.5 10 -5 c(H 3 O ) c(HCO ) 6.5 10 - 3 c(H 2 CO 3 ) 0.010mol L-1 -5 mol L -1 根据第二步解离计算c(CO 32- ): HCO - 3 (aq) H 2 O(l) ceq /( mol L1 ) 6.5 10-5 -y 6.5 10-5 y K a2 (H 2 CO 3 ) 4.7 10 H 3 O (aq) CO 32- (aq) y 11 5 {c(H 3 O )}{c(CO )} (6.5 10 y) y - 5 {c(HCO 3 )} 6.5 10 y 5 2- 3 5 6.5 10 y 6.5 10 , y K a2 4.7 10 c(CO 2- 3 1 ) K a2 mol L 4.7 10 11 11 1 mol L INORGANIC CHEMISTRY Section E2 pH value of salt solution (1) Strong acid + strong base compounds, neutral, pH =7. (2) weak acid + strong base compounds, the hydrolysis of the anion occurs. For example, (i) in NaAc solution Ac- + H2O HAc + OHKh = { [HAc][OH-]} / [Ac-] = Kw/Ka (II) NH4+ + 2 H2O NH3H2O + H3O+ Kh = { [NH3H2O][H3O+]} / [NH4+] = Kw/Kb (III) In NH4Ac solution Kh = Kw / (Ka Kb) INORGANIC CHEMISTRY Section E2 Problem 14.: Calculate the pH of 0.200 moldm-3 solution of NH4Cl. Solution.: Cl-, the conjugate base of a strong acid, does not react with water, but NH4+, the conjugate acid of NH3, does react: NH4+ + 2H2O NH3 H2O + H3O+ [ H 3O ][ NH 3 ] K w Kh Kb [ NH 4 ] Let [H3O+] = x, [NH3] = x, and [NH4+] = 0.200 -x 0.200 The solution of NH4Cl in water is acidic, as expected. K w 1.0 10 14 x2 Kh 5.6 10 10 [ H 3O ] x 1.1 10 5 5 0.200 K b 1.8 10 pH 4.96 INORGANIC CHEMISTRY Section E2 Buffer solution The pH value would keep stable if adding a little acid or base into the solution. The buffer solution consist of the conjugate acid-base pair. for example: NaAc- HAc, NH3H2O-NH4Cl, Na2HPO4-NaH2PO4, etc. In buffer solution (weak acid (HA)-salt (A-), HA + H2O A- CHA CA- [ A ][ H 3O ] Ka [ HA] K [ HA] K a C HA [ H 3O ] a [A ] C A O+ + H3 pH pKa lg For the weak base- salt system, [OH ] K bCbase Csalt pH 14 pK b lg Cbase Csalt Cacid C salt INORGANIC CHEMISTRY Section E2 Table I. Some common buffer system The agents The content of buffer solution pKa The range HCOOH-NaOH HCOOH-HCOO- 3.75 2.75 ~ 4.75 HAc-NaAc CH3COOH-CH3COO- 4.75 3.75 ~ 5.75 Na2B4O7 ~ HCl H3BO3 _ B(OH)4- 9.14 8.14 ~ 10.14 NH3 H2O-NH4Cl NH4+ - NH3 9.25 8.25 ~ 10.25 NaHCO3 – Na2CO3 10.25 9.25 ~ 11.25 HCO3- –CO32- INORGANIC CHEMISTRY Section E2 Problem 13.: Given 50 mL of 0.20 moldm-3 solution of the acid HA (Ka = 1.010-5) and 50 mL of an NaA solution. What should the concentration of the NaA solution be to make a buffer solution with pH = 4.00? Solution.: HA + H2O A- + H3O+ The addition of the two solutions halves the concentration of each solute assuming no reaction at all. The original NaA solution must be 2.0 10-2 moldm-3 to get this value of x after dilution. [ A ][ H 3O ] x 1.0 10 4 Ka 1.0 10 5 x 1.0 10 2 mol dm 3 [ HA] 0.10 INORGANIC CHEMISTRY Section E2 想配制pH=5.00 的缓冲溶液,需在50ml 0.10 mol·L-1的HAc溶液中加入 0.10 mol·L-1的NaOH多少毫升? 选择合适的酸,以pKa 接近所要求的pH值的酸最为适合。 Cacid pH pKa lg Csalt 5.00 4.75 lg x 32mL 50 0.10 0.10 x 0.10 x 例题:若在 50.00ml 0.150mol·L-1 NH3 (aq) 和 0.200 mol·L-1 NH4Cl组成的缓冲溶液中, 加入0.100ml 1.00 mol·L-1的HCl ,求加入HCl 前后溶液的pH值各为多少? 解:加入 HCl 前: c(B) pH 14 pK b lg c(BH ) 0.150 14 ( lg 1.8 10 ) lg 0.200 9.26 (0.12) 9.14 5 加入 HCl 后: 1.00 0.100 c(HCl) mol L1 0.0020mol L1 50.10 NH4 加HCl前浓 度/(mol·L-1) 加HCl后初始 浓度/(mol·L-1) NH3(aq) + H2O (l) 0.150 0.150-0.0020 (aq) +OH-(aq) 0.200 0.200+0.0020 平衡浓度 /(mol·L-1) 0.150-0.0020-x 0.200+0.0020+x x (0.202 x) x 5 1.8 10 0.148 x 5 5 1 x 1.3 10 c(OH ) 1.3 10 mol L pOH 4.89 pH 9.11 INORGANIC CHEMISTRY Section E2 Solvent Leveling effect Because the strong acid completely protolyzed, it is impossible to study this species itself in water. So the pH value would be the same for the strong acid with the same concentration and H3O+ is effectively the strongest acid possible there and any stronger acid is said to be leveled. Strong base would be leveled in water. HI, HCl, HBr, HNO3, HClO4 Solvent leveling limits the range of acid-base behavior that can be observed in given solvent, and is one reason for using other solvents with different leveling ranges. Liquid ammonia is very basic compared with water, and H2SO4 is very acidic. Differentiating solvent effect The strong & the weak acid in water (HCl, HAc) The strong & the weak base (NaOH,NH3H2O) HI,HBr,HCl ----in H2O, the acidity be leveled; in CH3OH, differentiated INORGANIC CHEMISTRY Section E2 Trend in pK value (1) AHn compounds, acid strength increases from left to right in the periodic table, for example, CH4 < NH3 < H2O << HF . This trend is most simply to related to the electronegativity increase of the element attached to hydrogen, which gives more bond polarity in the direction A- -H+ (2) Acid strength also increases down the group, HF < HCl < HBr < HI; because of the deceasing H-X bond strength down the group. (3) Oxides of nonmetallic elements, acids —— oxoacids in water (4) Oxides and hydroxides of metal, bases (5) Metals in high oxidation states can also form oxoacids INORGANIC CHEMISTRY Pauling’ s rules Section E2 INORGANIC CHEMISTRY Aqua cations Many metal cations are acidic in water. Salt containing these ions Al3+, Fe3+ form acidic solutions, and if the pH is increased successive protolysis may lead to polymerization and precipitation of an soluble oxide or hydroxide such as Al(OH)3. Some compounds show amphoteric behavior and dissolve in alkaline solution to give oxoanions. Al(OH)3 —— [Al(OH)4]- Section E2 INORGANIC CHEMISTRY Section E3 E3 Complex formation Introduction of complex Coordination compounds, complex A complex in general is any species formed by specific association of molecules or ions by donoracceptor interactions. A metal cation (Lewis acid)—— ligands. (Lewis base, ions and neutral molecules) The ligand acts as a donor and replaces one or more water molecules from the primary solvation sphere. Important terms: Coordination spheres: (内界): Counterions(抗衡离子,外界): Coordination bond, dative bond: steric isomerism Coordination numbers: coordination configuration isomerism(异构现象) Chelate(螯合,螯合物): Chelate ligands: Monodentate (unidentate); bidentate ; tridentate, tetradentate For example: en, ethylenediamine, EDTA (ethylenediamine tetraacetic acid) optical isomerism INORGANIC CHEMISTRY Section E3 en, ethylenediamine Phen EDTA 2, 2’- bpy Chelating ligands generally form stronger complexes than unidentate ones with similar donor properties. application: complexmetric titration,removing the toxic metals, etc. The most stable complexes generally being formed with four or five atoms, so that with the metal ion a five- or six- membered ring is formed INORGANIC CHEMISTRY Section E3 Macrocyclic compounds: macrocyclic effect Crown ether, & open-chain crown ether Cryptand ether, & open-chain ones Size selectivity: corresponding to different cavity sizes. e.g. Benzo-15-C-5, K+ & 6 + Na+ INORGANIC CHEMISTRY Section E3 Nomenclature of the complexes IUPAC nomenclature Follow the normal nomenclature of some inorganic compounds, (a) If the counterion is the simple anions, 某化某 (b) If the counterion is the complicate anions, 某酸某 (阴离子配体-中性配体-“合”-中心离子) For example: [Cu(NH3)4]SO4 :tetraaminecopper(II) sulfate;硫酸四氨合铜(II) K2[CuCl4] :potassium tetrachlorocuprate(II) (四氯合铜(II)酸钾) [Ni(NH3)6]Br2:hexaaminenickel(II) dibromide (溴化六氨合镍(II)) K[PF6]: potassium hexafluorophosphate (V) (六氟磷酸(V)钾) [Co(H2O)(NH3)3(Cl)2]Cl: dichloro-triamine-hydrate cobalt(III) chloride (氯化二氯三氨一水合钴(III)) INORGANIC CHEMISTRY Section E3 Equilibrium constants Stepwise formation constant, K1, K2, K3…….(逐级稳定常数) M + L ML ML + L ML2 [ ML ] [ ML2 ] K2 [ M ][ L ] [ ML ][ L ] K1 K 2 K 3 K1 Cumulative stability constant, 1, 2, 3……….(累积稳定常数) M + n L MLn n K1 K 2 K3 The bigger the equilibrium constants is, the higher the stability of the complex are. [Ag(NH3)2]+: 1.1×107 [Ag(CN)2]-: 1.3×1021 (for the same types ions) [Ag(SCN)2]-: 3.7×107 [Ag(S2O3)2]3-: 1.1×1013 NH3H2O: Na2S2O3,NaCN AgCl, AgBr, AgI 《近代化学导论》:P343 配离子的不稳定常数——配合物的稳定常数 INORGANIC CHEMISTRY Section E3 Hard and soft behavior Hard donors interact more strongly with hard acceptors, soft donors with soft acceptors (硬亲 硬,软亲软,软和硬的反应不容易发生 ). Hard acids tend to be more electropositive, and harder bases more electronegative. Softer donor and acceptor atoms tend to be larger and more polarizable. Class A(hard acid): the cations formed metals in early groups in periodic table Na+, K+, Mg2+,Al3+,etc. Class B(soft acid): some later transition metals and many post-transition metals Hg2+, Pb2+, Pt2+, Cd2+, etc. Class b forms strong complexes with ligands such as ammonia. But, Class a ions don’t complex with such ligands appreciably in water. Hard cations with low charge/size ratio, such as alkali ions, form very weak complexes with all ligands, except macrocycles. Some polyatomic ions such as NO3-, ClO4-, BF4-, PF6-, etc have very low complexing power to either class a or class b. ---- counterions for studying the thermodynamic properties of metal ions unaffected by complex formation. INORGANIC CHEMISTRY Section E4 E4 Solubility of ionic compounds Introduction of solubility Product When the ion compounds were dissolved in water, the following equilibrium will be found, MnXm(s) n Mm+ (aq) + n Xn-(aq) Equilibrium constant: the activities, not concentration K sp [M m ]n [ X n ]m Solubility product: (for dilute solutions) K sp ( PbI 2 ) [ Pb 2 ][ I ]2 2 K sp ( BaSO4 ) [ Ba 2 ][ SO4 ] 3 K sp (Ca3 ( PO4 ) 2 ) [Ca 2 ]3 [ PO4 ]2 AgCl Ksp= 1.77 ×10-7 AgCl > AgBr > AgI AgBr Ksp= 5.35 ×10-13 AgI Ksp= 8.51 ×10-17 INORGANIC CHEMISTRY Section E4 For the ionic compounds with different type, calculate the solubility from the solubility product. AgCl: Ksp= 1.77 ×10-7 Ag2CrO4 Ksp= 1.12 ×10-12 (1) AgCl ( s ) Ag (aq) Cl (aq) K sp ( AgCl ) [ Ag ][Cl ] [ Ag ]2 1.77 10 10 [ Ag ] K sp ( AgCl ) 1.33 10 5 mol dm 3 2 (2) Ag 2CrO4 ( s ) 2 Ag (aq ) CrO4 (aq ) 2 2 K sp ( Ag 2CrO4 ) [ Ag ]2 [CrO4 ] 4[CrO4 ]3 1.12 10 12 2 [CrO4 ] 3 K sp ( Ag 2CrO4 ) 4 1.04 10 4 mol dm 3 Solubility: AgCl < Ag2CrO4 INORGANIC CHEMISTRY Solubility product Principle (溶度积原理) It was widely applied to judge the trend and the extent for forming the precipitation from the mixture solution containing the Mm+ and Xn-ions. Firstly, calculate the J value (it is defined to be [Mm+]n[Xn-]m ) from the concentration of ions; Secondly, compare the relationship between the J value and the Ksp. If Ksp < J,the precipitation will be formed in solution and the equilibrium shifts to the direction of forming the precipitation. If Ksp > J,the precipitation will be dissolved and the equilibrium shifts to the direction of dissolving the precipitation to the solution. If Ksp = J,the equilibrium keep the balanced states. the rate of precipitating = the rate of dissolving Section E4 INORGANIC CHEMISTRY Section E4 Problem .: Determine whether a precipitate form in solution or not when Pb2+ solution (0.100 moldm-3, 100 mL ) is mixed with Cl- (0.30 moldm-3) in 100 mL solution. (Ksp(PbCl2) = 1.7 10-4 ) Solution.: Assuming that no precipitate form and the final volume keep to be 200 mL, the lead ion and chloride ion concentrations would be 0.005 and 0.15 moldm-3, respectively. PbCl2 Pb2+ + Cl- Ksp(PbCl2) = [Pb2+][Cl-]2 = 1.7 10-4 But if there were no precipitation, [Pb2+][Cl-]2 = (0.005)(0.15)2 = 1.1 10-3. For this product is much greater than the value of Ksp(PbCl2), the concentration of ions exceed that required for equilibrium, and the precipitation will form in the mixture. INORGANIC CHEMISTRY Section E4 The influence of pH value in solution Problem .:Whether Mg(OH)2 precipitate or not in a solution containing Mg2+ (0.0010 moldm-3) if the OH- concentration of the solution were altered to be (a) 10-5 moldm3 (b) 10-3 moldm-3 ? (K (Mg(OH) ) = 1.2 10-11) sp 2 Answer.: Ksp = [Mg 2+][OH-]2 = 1.2 10-11 [OH-]2 = 1.2 10-11/ [Mg 2+] = 1.2 10-8 [OH-]sat = 1.1 10-4 moldm-3 (a) [OH-] does not exceed [OH-]sat, so no precipitation will be observed. (b) [OH-] exceeds [OH-]sat, so Mg(OH)2 will precipitate from the solution. Application: The selectivity separation of metal ions in mixture solution would be successfully finished by controlling the pH value. INORGANIC CHEMISTRY Section E4 The influence of pH value in solution The basis of control the pH value of solution to separate the metal ions: (a) the condition of forming the precipitation in solution: J > Ksp (b) the condition for judging whether the metal ion precipitate completely or not: [Mn+] < 10-5 moldm-3 M (OH ) n ( s ) M n (aq) OH (aq) K sp ( M (OH ) n ) [ M n ][OH ]n [OH ] n pH 14 K sp [M n ] K sp 1 lg n [M n ] 例题:在含有0.10mol·L-1 Fe3+和 0.10mol·L1 Ni2+的溶液中,欲除掉Fe3+,使Ni2+仍留 在溶液中,应控制pH值为多少? 解: K sp 开始沉淀pH 沉淀完全pH 16 Ni(OH) 2 5.0 10 39 Fe(OH) 3 2.8 10 6.85 Fe3+沉淀完全 2.82 c(Fe3+)≤10-5 2.82 Ni2+开始沉淀 6.85 可将pH值控制在 2.82 ~ 6.85 之间 pH INORGANIC CHEMISTRY Section E4 common ion effect MnXm(s) n Mm+ (aq) + n Xn-(aq) Adding one of the ions Mm+ or Xn- will shift the equilibrium to the left and so reduce the solubility of the salt. For example, calculate the solubility of AgCl(s) in HCl (1.0×10-2 moldm-3) solution. (1) Before adding HCl, [Ag+]=1.33×10-5 moldm-3 AgCl (s) Ag+(aq) + Cl-(aq) (2) After adding HCl, x x x+0.010 K sp ( AgCl) [ Ag ][Cl ] ( x)( x 0.010) 1.77 1010 x 0.010mol dm 3 , x 0.010 0.01mol dm 3 x 1.77 108 mol dm 3 INORGANIC CHEMISTRY Section E4 Complexing effect If adding some compound able to complex the insoluble salt, its solubility would markedly rise. For example: Calculate the solubility of AgCl in NH3H2O (6 moldm-3) sultion. Answer.: Initial state (moldm-3) Balanced state(moldm-3) AgCl (s) + NH3H2O [Ag(NH3)2]+(aq) + Cl-(aq) + 2 H2O 6 0 0 6 - 2x x x Ksp(AgCl) =1.7×10-10, K{[Ag(NH3)2]+}=1.7×107 [ Ag ( NH 3 ) 2 ] [Cl ] [ Ag ( NH 3 ) 2 ] K [ Ag ][Cl ] 2 2 [ NH 3 ] [ NH 3 ] [ Ag ] K sp ( AgCl ) K{[ Ag ( NH 3 ) 2 ] } (1.7 10 10 ) (1.6 107 ) 2.7 10 3 x2 2.7 10 3 x 0.283mol dm 3 2 (6 2 x ) INORGANIC CHEMISTRY Section E4 Thermodynamic Analysis Go =-RT lnKsp (a) the formation of gas-phase ions; (b) their subsequent solvation Total energy: (a) lattice energy of the solid (b) solvation enthalpies of the ions With ions of very different size, solvation is relatively favored and solubility tends to be larger than with ions of similar size. INORGANIC CHEMISTRY Section E4 Major trends in Water (i) Soluble salts are more often found when ions are of very different size rather than similar size. (a) For example: different alkali metal cations: OH- & F- + Li+ -------least soluble Cl- & NO3- —— soluble (b) precipitate a large complex ions, a large cation such as [Bu 4N]+ can be helpful. (ii) Salts where both ions have multiple charges are less likely to be soluble than ones with single charges. CO32- & SO42-, insoluble (iii) With ions of different charges, especially insoluble compounds result when the lower charged one is smaller. M3+ + fluorides and hydroxides: very insoluble + heavier halide or nitrates: very soluble. (iv) Lower solubility result from covalent contributions to the lattice energy. —— with ions of less electropositive metals in combination with more polarizable cations. Late transition and post-transition elements + S2- —— insoluble; X- —— insoluble. INORGANIC CHEMISTRY Section E4 Influence of pH and complexing Any substance in solution that reacts with one of the ions formed the following reaction will shift the equilibrium to the right and hence increase the solubility of the solid. MnXm(s) n Mm+ (aq) + n Xn-(aq) (a) pH value will therefore influence the solubility in a range where one of the ions has significant Brnsted acid or base properties. The anion is the conjugate base of the weak acid solubility will increase at low pH. MxOy, MCO3, MS, M(OH)n.—— dissolve in acid solution, and conversely such solids may be precipitated from a solution containing a metal ions as the pH is increased. Amphoteric: (b) The conjugate acid of the anion is volatile, or decomposes to form a gas. MCO3 & MS (c) Any ligand that complexes with metal ion will also increase solubility. INORGANIC CHEMISTRY Section E5 E5 Electrode Potentials Standard potentials Electrochemical cell: Two Electrodes: anode (reduction reaction ) and cathode (oxidation reaction) half reaction: Zn + Cu2+ = Cu + Zn2+ -- cell reaction (+) Cu2+ + 2e Cu ----half reaction (-) Zn - 2e Zn2+ 氧化型 Z e Zn 2 /Zn 还原型 , Cu 2 /Cu () Zn Zn 2 (1.0mol L1 ) ‖ Cu 2 (1.0mol L1 ) Cu () INORGANIC CHEMISTRY Section E5 书写原电池符号的规则: ①负极“-”在左边,正极“+”在右边, 盐桥用“‖”表示。 ②半电池中两相界面用“ ”分开,同 相不同物种用“,”分开,溶液、气体要注明 cB,pB 。 ③纯液体、固体和气体写在惰性电极一 边用“,”分开。 INORGANIC CHEMISTRY Section E5 例:将下列反应设计成原电池并以原电池符号表示。 2Fe 1.0mol L Cl2 101325Pa 2 1 3 1 1 2Fe 0.1mol L 2Cl 2.0mol L 正 极 Cl 2 (g ) 2e 2 负 极 Fe (aq ) e 2 1 3 2Cl (aq ) 3 Fe (aq ) 1 () Pt Fe 1.0mol L , Fe 0.1mol L ‖ Cl 2.0mol L 1 Cl2 101325Pa ,Pt () INORGANIC CHEMISTRY Section E5 In a electrochemical cell, a redox reaction occurs in two halves. Electrons are liberated by the oxidation half reaction at one electrode and pass through an electrical circuit to another electrode where they are used for the reduction. Cell potential: is the potential difference between the two electrodes required to balance the thermodynamic tendency for reaction, so that the cell is in equilibrium and no electrical current flows. Go = - nFE = - RT lnK (F---- the Farady constant, 96500 cmol-1, n--- the number of moles of electrons passed per mole of reaction) --------- measurable Electrode potential: ----- not measurable Standard electrode potential.: The potential of standard hydrogen electrode is defined as zero at standard state. ([H+] = 1moldm-3, PH2= 100 kPa) H+ + e ½ H2 INORGANIC CHEMISTRY Section E5 Only differences in electrode potential are significant, the absolute values having no meaning except in comparison with H+ / H2 potentials. INORGANIC CHEMISTRY Section E5 Direction of reaction Comparing two couples Ox-Red, a more positive means that the corresponding species Ox is a stronger oxidizing agent. 0 (electrode potential) Ox1 + ne Red 1 big Ox2 + ne Red 2 samll Ox1 + Red 2 Red 1 + Ox2 the positive reaction-------- very easy the reverse reaction--------- hard Go = - nFE = - RT lnK (K ---equilibrium constant) nFE ln K RT E 0 0 0 例:判断在酸性溶液中H2O2与Fe2+混合时,能否发生氧化还原反应?若能反应, 写出反应方程式。 3 Fe ( aq ) e 解: 2 Fe (aq) 2e O2 (g) 2H (aq) 2e 2 Fe (aq) Fe(s) 0.769V 0.4089V H2O2 (aq) 0.6945V 2H2O(l) 1.763V H2O2 (aq) 2H (aq) 2e 2 H2O2与 Fe 发生的反应: 2 H2O2 (aq) 2Fe (aq) 2H (aq) 3 2Fe (aq) 2H2O(l) 3 2 MF (H2O2 / H2O) (Fe / Fe ) 1.763V 0.769V 0.994V 0.2V INORGANIC CHEMISTRY Section E5 Non-standard conditions Under nonstandard conditions the electrode potential of a couple can be calculated from the Nernst equation: RT [Ox ] 0.0592 [Ox ] ln 0 lg nF [Re d ] n [Re d ] RT [Ox ] 0.0592 [Ox ] E E0 ln E0 lg nF [Re d ] n [Re d ] 0 (1) When a reaction involves H+ or OH- ions, these must be included in the Nernst equation to predict the pH dependence of the couple. 4 MnO 8H 5e MnO /Mn 4 2 Mn 4H 2 O 2 4 8 0.0592V {c(MnO )}{c(H )} lg (MnO /Mn ) 2 5 {c(Mn )} 4 2 ② 介质的酸碱性 3 例: 已知 EA (ClO /Cl ) 1.45V 3 1 求:当c(ClO ) c(Cl ) 1.0mol L , 1 c(H ) 10.0mol L 时,E (ClO3 /Cl ) ? 解:ClO3 (aq) 6H (aq) 6e 3 Cl (aq) 3H2O(l) A (ClO /Cl ) 3 6 0.0592V {c(ClO )}{c(H )} A (ClO /Cl ) lg 6 {c(Cl )} 0.0592V 6 1.45V lg10.0 1.51V 6 3 ③沉淀的生成对电极电势的影响 例: 已知 (Ag / Ag) 0.799V,若在Ag 和Ag组成的半电池中加入NaCl会产生AgCl s , 1 当 c(Cl ) 1.0mol L 时, (Ag / Ag) ? 并求 10 (AgCl/ Ag) ? ( Ksp (AgCl) 1.8×10 ) Ag Ag c(Cl ) 1.0mol L1 Ag (aq ) Cl (aq ) 解:AgCl (s) {c( Ag )}{ c(Cl )} Ksp (AgCl) 1 若c(Cl ) 1.0mol L 时 , c( Ag ) Ksp (AgCl) Ag (aq ) e Ag(s) ( Ag / Ag) ( Ag / Ag) 0.0592V lg {c( Ag )} ( Ag / Ag) 0.0592V lg Ksp ( AgCl) 0.799V 0.0592V lg 1.8×10 0.222V 10 INORGANIC CHEMISTRY Section E3 (2) Complex formation: In general, any ligand that complexes more strongly with the higher oxidation state will reduce the potential. Conversely, the potential increases if the lower oxidation state is more strongly complexed. 2 例 :已知 (Cu /Cu ) 0.3394V , 2 12 2 K f (Cu(NH 3 ) 4 ) 2.30×10 。在 Cu /Cu 半 电 1 池 中,加入氨水,当 c ( NH 3 ) 1.0mol L , 2 3 4 1 2 c (Cu(NH ) ) 1.0mol L 时, (Cu /Cu ) ? 2 3 4 并求 (Cu(NH ) / Cu ) ? Cu c( NH3 ) c(Cu(NH 3 ) 24 ) 1.0mol L1 Cu 2 氨水 解:Cu 2 (aq) 4NH 3 (aq) 2 3 4 Cu(NH ) (aq) 2 3 4 {c(Cu(NH ) )} Kf 2 4 {c(Cu )}{c( NH 3 )} 2 3 4 1 当 c( NH 3 ) c(Cu(NH ) ) 1.0mol L 时 1 2 c(Cu ) 2 K f (Cu(NH 3 ) 4 ) 2 Cu (aq) 2e Cu(s) 2 (Cu / Cu) 0.0592 2 (Cu / Cu) lg{c(Cu )} 2 0.0592 1 2 (Cu / Cu) lg 2 2 K f (Cu(NH 3) 4 ) 2 0.0592 1 0.3394V lg 12 2 2.30×10 0.0265V 2 3 4 Cu(NH ) (aq) 2e Cu(s) 4NH 3(aq) 1 2 当 c (NH3 ) c(Cu(NH3 ) 4 ) 1.0mol L 时 , 2 3 4 2 (Cu(NH ) / Cu) (Cu /Cu) 0.0265V 2 3 4 即 (Cu(NH ) / Cu) 0.0592V 1 2 (Cu /Cu) lg 2 2 K f ( Cu(NH3) 4 ) 2 3 4 2 (Cu(NH ) / Cu) (Cu /Cu) INORGANIC CHEMISTRY Section E5 Diagrammatic Representation: Diagrammatic Representation: (a) Latimer diagram: shows the standard electrode potentials associated with the different oxidation states of an element.(元素电极电势图) (b) Frost or oxidation state diagram 1.判断歧化反应能否发生 2 2Cu (aq) Cu(s) Cu (aq) 0.1607V 0.5180V 2 Cu / V Cu 0.3394V (Cu / Cu) 2 (Cu / Cu ) 0.5180V 0.1607V 0.3573 V 0 右 左 发生歧化反应; 右 左 发生歧化逆反应。 Cu 2.计算电对的电极电势 (Hess’ Law) E1 E2 E3 A B C D (Z1) (Z3) (Z2) Ex (Zx) A Z1e B Z 2e +)C Z3e A Z xe B 1 r Gm(1) Z1 F 1 C 2 r Gm(2) Z 2 F 2 D 3 r Gm(3) Z 3 F3 D x r Gm(x ) Zx F x Z x Z1 Z 2 Z 3 r Gm( x ) r Gm(1) r Gm(2) r Gm(3) Z x F Z 1 F 1 Z 2 F 2 Z 3 F 3 x Z1 1 Z 2 2 Z 3 3 Z1 1 Z 2 2 Z 3 3 x Zx INORGANIC CHEMISTRY G10 G20 G30 3 0 ( Mn 3 / Mn ) 0 ( Mn 3 / Mn 2 ) 2 0 ( Mn 2 / Mn) 0 ( Mn 3 / Mn ) 0.29V Section E5 例题:已知Br的元素电势图如下 E2 3 BrO E1 BrO 0.4556 Br2 1.0774 Br E3 0.6126 (1) 求 1 、 2 和 3 。 (2)判断哪些物种可以歧化? (3) Br2(l)和NaOH(aq)混合最稳定的产物是 什么?写出反应方程式并求其 K 。 E2 解:(1) 3 BrO E1 BrO 0.4556 Br2 1.0774 Br E3 0.6126 (0.6126 6 0.4556 1 1.0774 1)V E1 0.5357V 4 (0.4556 1 1.0774 1)V E2 0.7665V 2 (0.6126 6 1.0774 1)V E3 0.5196V 5 (2) 0.7665 3 BrO 0.5357 BrO 0.4556 0.5196 Br2、BrO 可以歧化。 Br2 1.0774 Br (3) 因为 BrO 能歧化 ,不稳定, 所以 Br 2 (l) 与 NaOH 混合最稳定的产物 3 是 BrO 和 Br 。 3Br 2 ( l ) 6OH ( aq ) E MF 3 5Br ( aq ) BrO ( aq ) 3H 2 O(aq) E (Br 2 /Br ) E (BrO3 /Br 2) 1 . 0774V 0.5196V 0 . 5578 V ZE MF 5×0.5578V 47 . 11 lg K 0.0592V 0.0592V 47 K 1 . 29×10 INORGANIC CHEMISTRY Section E5 Frost or oxidation state diagram The potential 0 in volts between any two oxidation states is equal to the slope of the line between the points in this diagram. ] Steep positive slopes show strong oxidizing agents, steep negative slopes strong reducing agents. INORGANIC CHEMISTRY Section E5 Application: (a) Display the comparative redox properties of elements. (b) Provide a visual guide to when disproportionation of a species is expected. pH=0, Mn3+ (aq) + 2 H2O MnO2 (s) + Mn2+ (aq) + 4 H+ (aq) Mn(V) Mn(VI) INORGANIC CHEMISTRY Section E5 Kinetic limitations Electrode potentials are thermodynamic quantities and show nothing about how fast a redox reaction can take place. Simple electron transfer reactions are expected to be rapid, but redox reactions where covalent bonds are made or broken may be much slower. MnO4-/MnO2 + H2O/ O2 Kinetic problems can also affect redox reactions at electrodes when covalent substances are involved. f f 作业: 6, 7, 9, 11, 12. INORGANIC CHEMISTRY Sec. F Section F Introduction to Non-metals Covalent chemistry (1) Hydrogen and boron stand out in their chemistry. Hydrogen —— one covalent bond. Boron —— electron deficiency (2) In the other elements, valence states depend on the electron configuration and on the possibility of octet expansion which occurs in period 3 onwards. octet rules—— octet expansion (3) Multiple bonds are common in period 2 but are often replaced by polymerized structures with heavier elements. C, N,O INORGANIC CHEMISTRY Section F Ionic chemistry Simple anionic chemistry is limited to oxygen and the halogens, although polyanions and polycations can be formed by many elements. Acid-base chemistry Many halides and oxides are Lewis acids; BCl3 SO3 The compounds with lone-pairs are Lewis bases; NH3,H2O Brnsted acidity is possible in hydrides and oxoacids. Halide complexes can be also formed by ion transfer. Redox Chemistry The oxidizing power of elements and their oxides increases with group number. Vertical trends show an alternation in the stability of the highest oxidation states. O, F, Br, Cl. INORGANIC CHEMISTRY Sec. G Section G Introduction to Non-transition metals Scope Non-transition metals includes groups 1 to 2 of the s-block elements, group 12 and pblock elements in lower periods. (a) Aluminum and the elements of groups 1 and 2 are classed as pre-transition metals, —— typical metals, very electropositive in character and almost invariably found oxidation states expected for ions in a noble-gas configuration. —— found widely in silicate minerals (b) The remaining ones (metallic elements from periods 4-6 in groups following the transition series) as post-transition metals. —— less electropositive than the pre-transition metals and are typically found nature as sulfides rather than silicates. in solution, post-transition metals less ionic in character than corresponding compounds of pre-transition metals; In solution, post-transition metals form stronger complexes than pretransition metals. lower oxidation states. (the sixth period, Tl+, Pb2+, Bi3+) INORGANIC CHEMISTRY Section G Positive ions Formation of compounds with positive ions depends on a balance between ionization energies and lattice or solvation energies. Post-transition metals have higher ionization energies and are less electropositive than pre-transition metals. Group trends Trends down groups 1 and 2 are dominated by increasing ionic size. In later groups the structural and bonding are less regular and there is an increased tendency to lower oxidation states, especially in period 6. Non-cationic chemistry Many of the elements can form anionic species. Compounds with covalent bonding are also known: these include organometallic compounds, and compounds containing metal-metal bonds( especially with post-transition metals). INORGANIC CHEMISTRY Sec. H Section H Introduction to transition-metals Scope Transition elements form groups 3-11 in the d block. They have distinct chemical characteristic resulting from the progressive filling of the d shells. These includes the occurrence of variable oxidation states, and compounds with structures and physical properties resulting from partially filled d orbitals. Typical transition metal characteristics include: (a) the possibility of variable oxidation states; (b) Compounds with spectroscopic, magnetic or structural features resulting from partially occupied d orbital. (c) An extensive range of complexes and organometallic compounds including ones with very low oxidation state (zero or even negative) (d) Useful catalytic properties shown by metals and by solid or molecular compounds INORGANIC CHEMISTRY Section H Vertical trends Elements of the 3d series are chemically very different from those in the 4d and 5d series, showing weaker metallic and covalent bonding, stronger oxidizing properties in high oxidation states and the occurrence of many more compounds with unpaired electrons. Horizontal trends Electropositive character declines towards the right of each series. Elements become less reactive and their compounds show a tendency towards softer behavior. Later elements in the 4d and 5d series are relatively more inert. INORGANIC CHEMISTRY Section H Electron configuration Neutral atoms have both s and d valence electrons, but in chemically important states are often regarded as having purely d configuration. In the 3d series most atoms have the configuration (3d)n(4s)2, where n increase from one to 10; chromium and copper are however exceptions with (3d)5(4s)1 and (3d)10(4s)1 respectively. The configurations depend on a balance of two factors: (a) 3d orbitals are progressively stabilized relative to 4s across the series; (b) Repulsion between electrons is large in the small 3d orbitals, and so minimum energy in the neutral atom is achieved in spite of (a) by putting one or two electrons in the 4s orbitals. In forming positive ions, 4s electrons are always removed first. For M2+ ions and ones of higher charge, outer shell electrons are left only in the 3d series. INORGANIC CHEMISTRY Sec. I Section I Lanthanum and lanthanides The elements (1) The elements (sometimes called rare earths) are found together in nature and are electropositive metals. —— with the filling of seven orbitals of the 4f shells. Ln (lanthanide, 镧系元素) ——La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu RE(rare earth) —— La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu +Sc, Y (2) Chemistry is dominated by +3 state with ions in (4f)n configuration, and is similar for all elements. Ligand field and chemical bonding effects associated with incomplete 4f orbitals are very small and hardly detectable in chemical trends. The chemistry of all Ln3+ ions is very similar and differentiated only by the gradual contraction in radius associated with increasing nuclear charge. The lanthanide contraction(镧系收缩效应): +2, +4 INORGANIC CHEMISTRY Section I Oxidation States +3 A wide range of +3 compounds is formed as well as aqua ions. The ionic radius decrease gradually across the series, leading to changes in solid structures, and an increase in stability of complexes in solution. Organometallic compounds are more ionic than in the d block. Ln3+ : halides, LnX3, oxides, Ln2O3 this relatively large size for 3+ ions is associated with correspondingly high coordination numbers in solid compounds. LnF3 (CN=9), Ln2O3. (CN=7)for earlier elements. For later Ln elements the decrease in radius leads to changes in structure with reduction in coordination. The aqua Ln3+ ions show slightly acidity, which increases from La to Lu as the radius decrease but still much less than for Al3+. Strong complexes are formed with hard oxygen donor ligands, and especially chelating ones such as EDTA, or -diketones. Complexes strengths generally increase across the series as the radius decreases, and this may be used to separate a mixture of Ln 3+ ions. INORGANIC CHEMISTRY Section I Other Oxidation States Sm, Eu and Yb form many compounds in the +2 oxidation state. With the other elements, compounds in this state are formed only with large anions and are often metallic. LnS, LnI2 Ce, and to a lesser extent Pr, and Tb show the +4 state. CeO2, Pr6O11, Tb4O7 ( Tb(IV) )