Chemistry MCQS 12 class
... 18. The atoms of the elements belonging to the same period of the Periodic table have __________. (Same number of protons, same number of neutrons, same number of valence shells) 19. Sodium thiosulphate is used in photography because of its __________. ...
... 18. The atoms of the elements belonging to the same period of the Periodic table have __________. (Same number of protons, same number of neutrons, same number of valence shells) 19. Sodium thiosulphate is used in photography because of its __________. ...
08 Redox Reactions
... In binary compounds of metals and non-metals the oxidation number of metals is always positive while that of non-metals is negative. Eg. In NaCl, the oxidation number of sodium is + 1 and that of chlorine is 1. In compounds formed by the combination of non-metallic atoms, the atom with higher elec ...
... In binary compounds of metals and non-metals the oxidation number of metals is always positive while that of non-metals is negative. Eg. In NaCl, the oxidation number of sodium is + 1 and that of chlorine is 1. In compounds formed by the combination of non-metallic atoms, the atom with higher elec ...
PS 2 - Purdyphysicalscience
... Use the atomic number and the mass number to calculate the number of protons, neutrons, and/or electrons for a given isotope of an element Predict the charge that a representative element will acquire according to the arrangement of electrons in its outer energy level Compare fission and fusion (inc ...
... Use the atomic number and the mass number to calculate the number of protons, neutrons, and/or electrons for a given isotope of an element Predict the charge that a representative element will acquire according to the arrangement of electrons in its outer energy level Compare fission and fusion (inc ...
File
... commonly thought of as a metal, does have some nonmetallic properties as its bonds to other nonmetals have significant covalent character. The other Group 3A elements have typical metal characteristics; its compounds formed with nonmetals are ionic. From this discussion, metallic character increases ...
... commonly thought of as a metal, does have some nonmetallic properties as its bonds to other nonmetals have significant covalent character. The other Group 3A elements have typical metal characteristics; its compounds formed with nonmetals are ionic. From this discussion, metallic character increases ...
CH_3 IN TOTO - WordPress.com
... Atomic mass is the • weighted average of all naturally occurring isotopes of that element • number on the periodic table below the chemical symbol with two decimal places ...
... Atomic mass is the • weighted average of all naturally occurring isotopes of that element • number on the periodic table below the chemical symbol with two decimal places ...
D--All Websites-eChemistryHelp-.mdi
... 1. The definition : Oxidation number of an element in a particular compound represents the number of electrons lost or gained by an element during its change from free state into that compound or Oxidation number of an element in a particular compound represents the extent of oxidation or reduction ...
... 1. The definition : Oxidation number of an element in a particular compound represents the number of electrons lost or gained by an element during its change from free state into that compound or Oxidation number of an element in a particular compound represents the extent of oxidation or reduction ...
Preview Sample 2
... 21. Which of the following is the same for isotopes of an element? A. mass number B. mass of an atom C. neutron number D. atomic number E. both atomic number and neutron number 22. Which of the following statements about isotopes is incorrect? A. The isotopes of an element have the same number of pr ...
... 21. Which of the following is the same for isotopes of an element? A. mass number B. mass of an atom C. neutron number D. atomic number E. both atomic number and neutron number 22. Which of the following statements about isotopes is incorrect? A. The isotopes of an element have the same number of pr ...
101
... To assign oxidation numbers to the atoms in a water molecule, you can consider all the bonding electrons to be “owned” by the more electronegative oxygen atom, as shown in Figure 10.4B. Thus, each hydrogen atom in a water molecule is considered to have no electrons, as hydrogen would in a hydrogen ...
... To assign oxidation numbers to the atoms in a water molecule, you can consider all the bonding electrons to be “owned” by the more electronegative oxygen atom, as shown in Figure 10.4B. Thus, each hydrogen atom in a water molecule is considered to have no electrons, as hydrogen would in a hydrogen ...
Chemistry Atoms, Molecules, and Ions 2.1 Multiple
... B) Rutherford's gold foil experiment. C) Thomson's cathode ray tube experiment. D) None of these Answer: B Topic: Section 2.4 Atomic Structure: Protons and Neutrons 18) The existence of neutrons in the nucleus of an atom was demonstrated by A) Millikan's oil drop experiment. B) Rutherford's gold foi ...
... B) Rutherford's gold foil experiment. C) Thomson's cathode ray tube experiment. D) None of these Answer: B Topic: Section 2.4 Atomic Structure: Protons and Neutrons 18) The existence of neutrons in the nucleus of an atom was demonstrated by A) Millikan's oil drop experiment. B) Rutherford's gold foi ...
Into the Atom - Structure of the Nucleus, Heavy Nuclei... Original Script and research: Dr. Arvind Dubey
... Bang which occurred approximately 13.7 billion years ago. During the following three minutes most of the helium, lithium, and deuterium are produced in the universe, and perhaps some of the beryllium and boron. My first ancestor, complete with bound electrons was created 380,000 years after the Big ...
... Bang which occurred approximately 13.7 billion years ago. During the following three minutes most of the helium, lithium, and deuterium are produced in the universe, and perhaps some of the beryllium and boron. My first ancestor, complete with bound electrons was created 380,000 years after the Big ...
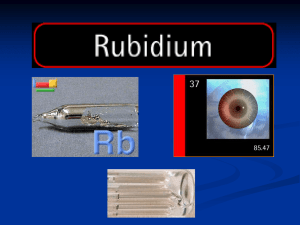
Rubidium
... It occurs naturally in the minerals leucite, pollucite, and zinnwaldite, which contains traces of up to 1% of its oxide. Lepidolite contains 1.5% rubidium and this is the commercial source of the element. Some potassium minerals and potassium chlorides also contain the element in commercially signif ...
... It occurs naturally in the minerals leucite, pollucite, and zinnwaldite, which contains traces of up to 1% of its oxide. Lepidolite contains 1.5% rubidium and this is the commercial source of the element. Some potassium minerals and potassium chlorides also contain the element in commercially signif ...
FREE Sample Here
... Full file at http://testbank360.eu/test-bank-chemistry-atoms-first-2nd-edition-burdge ...
... Full file at http://testbank360.eu/test-bank-chemistry-atoms-first-2nd-edition-burdge ...
The d-Block Elements
... (χ = 1.4) to Cu (χ = 1.9). Thus Sc is a rather active metal, whereas Cu is much less reactive. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E° for the reaction M2+(aq) + 2e− → M0(s) becomes progressively less negative from Ti (E° = −1.63 V) to ...
... (χ = 1.4) to Cu (χ = 1.9). Thus Sc is a rather active metal, whereas Cu is much less reactive. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E° for the reaction M2+(aq) + 2e− → M0(s) becomes progressively less negative from Ti (E° = −1.63 V) to ...
Chem 11 Review Answers - hrsbstaff.ednet.ns.ca
... 8. What must be true about the spins of two electrons occupying the same orbital? ...
... 8. What must be true about the spins of two electrons occupying the same orbital? ...
Chapter 2 - Atoms and the Periodic Table (test bank)
... In the early 1900s, Ernest Rutherford performed an experiment with thin foils of gold and alpha particles to probe the structure of the atoms. He observed that most of these alpha particles penetrated the foil and were not deflected. Realizing that atoms are electrically neutral (that is, they have ...
... In the early 1900s, Ernest Rutherford performed an experiment with thin foils of gold and alpha particles to probe the structure of the atoms. He observed that most of these alpha particles penetrated the foil and were not deflected. Realizing that atoms are electrically neutral (that is, they have ...
RedOx notes:
... Which elements have specific rules? Which element(s) do(es) not have rules? Use rule 8 or 9 from above to calculate these. ...
... Which elements have specific rules? Which element(s) do(es) not have rules? Use rule 8 or 9 from above to calculate these. ...
RedOx notes:
... Which elements have specific rules? Which element(s) do(es) not have rules? Use rule 8 or 9 from above to calculate these. ...
... Which elements have specific rules? Which element(s) do(es) not have rules? Use rule 8 or 9 from above to calculate these. ...
FREE Sample Here - We can offer most test bank and
... a. law of conservation of energy c. law of constant composition b. law of conservation of mass d. all of the above ANS: C PTS: 1 TOP: 2.3 - WHAT ARE THE POSTULATES OF DALTON’S ATOMIC THEORY? 26. Which of the following statements, all of which were part of Dalton’s atomic theory, was later shown to b ...
... a. law of conservation of energy c. law of constant composition b. law of conservation of mass d. all of the above ANS: C PTS: 1 TOP: 2.3 - WHAT ARE THE POSTULATES OF DALTON’S ATOMIC THEORY? 26. Which of the following statements, all of which were part of Dalton’s atomic theory, was later shown to b ...
View PDF
... Rutherford fired positively charged particles at metal foil and concluded that most of the mass of an atom was a. in the electrons. c. evenly spread throughout the atom. b. ...
... Rutherford fired positively charged particles at metal foil and concluded that most of the mass of an atom was a. in the electrons. c. evenly spread throughout the atom. b. ...
Atoms and Elements
... that surface (see Figure 2.2(a) 왔). In other words, Binnig and Rohrer had discovered a type of microscope that could “see” atoms. When I was a child taking science in elementary school, my teachers always told me that, although scientists were certain that matter was made of atoms, we could not see ...
... that surface (see Figure 2.2(a) 왔). In other words, Binnig and Rohrer had discovered a type of microscope that could “see” atoms. When I was a child taking science in elementary school, my teachers always told me that, although scientists were certain that matter was made of atoms, we could not see ...
2 Atoms and Molecules
... In Chapter 1, we defined elements as homogeneous pure substances made up of identical atoms. At least 115 different elements are known to exist. This leads to the conclusion that a minimum of 115 different kinds of atoms exist. Eighty-eight of the elements are naturally occurring and therefore are f ...
... In Chapter 1, we defined elements as homogeneous pure substances made up of identical atoms. At least 115 different elements are known to exist. This leads to the conclusion that a minimum of 115 different kinds of atoms exist. Eighty-eight of the elements are naturally occurring and therefore are f ...
Introduction to chemistry Multiple Choice 1. Which SI prefix means
... substance B. Therefore, the density of substance A is lower than the density of substance B. Answer: True; Difficulty: hard; Reference: Section 2.9 100. Two substances A and B have the same mass. Substance A occupies half the volume of substance B. Therefore, the density of substance A is lower than ...
... substance B. Therefore, the density of substance A is lower than the density of substance B. Answer: True; Difficulty: hard; Reference: Section 2.9 100. Two substances A and B have the same mass. Substance A occupies half the volume of substance B. Therefore, the density of substance A is lower than ...
Atoms: The Building Blocks of Matter - Milton
... Democritus’s idea into a scientific theory that could be tested by experiment. But not all aspects of Dalton’s atomic theory have proven to be correct. For example, today we know that atoms are divisible into even smaller particles (although the law of conservation of mass still holds true for chemi ...
... Democritus’s idea into a scientific theory that could be tested by experiment. But not all aspects of Dalton’s atomic theory have proven to be correct. For example, today we know that atoms are divisible into even smaller particles (although the law of conservation of mass still holds true for chemi ...