chapter 4 types of chemical reactions and solution
... a. Volumes are always estimated to one position past the marked volume increments. The estimated volume of the first beaker is 32.7 mL, the estimated volume of the middle beaker is 33 mL, and the estimated volume in the last beaker is 32.73 mL. b. Yes, all volumes could be identical to each other be ...
... a. Volumes are always estimated to one position past the marked volume increments. The estimated volume of the first beaker is 32.7 mL, the estimated volume of the middle beaker is 33 mL, and the estimated volume in the last beaker is 32.73 mL. b. Yes, all volumes could be identical to each other be ...
Synthesis, Structural Variations and Trends In the Properties of
... In these units the divalent metal centers are also linked by a small anionic bridge, X = F–, Cl–, Br–, OH–, CN-, N3-, to generate [M2(–X)(–L)2]3+, where L = Lm or Lm*. The tendency of these ligands to form a single structural type prompted us to tackle a fundamental problem in magnetism: carefully ...
... In these units the divalent metal centers are also linked by a small anionic bridge, X = F–, Cl–, Br–, OH–, CN-, N3-, to generate [M2(–X)(–L)2]3+, where L = Lm or Lm*. The tendency of these ligands to form a single structural type prompted us to tackle a fundamental problem in magnetism: carefully ...
Answers to SelectedTextbook Questions
... (e) Gibbs free energy, G = H − TS, combines enthalpy and entropy to give a quantity which must decrease for any processes that actually happens. (f) Lewisite is a chlorinate alkyl arsenic compound which was produced as a chemical weapon causing blisters and lung irritation. (g) A Lewis base ...
... (e) Gibbs free energy, G = H − TS, combines enthalpy and entropy to give a quantity which must decrease for any processes that actually happens. (f) Lewisite is a chlorinate alkyl arsenic compound which was produced as a chemical weapon causing blisters and lung irritation. (g) A Lewis base ...
Question Bank (Class XI - Chemistry)
... Ans. Matter can neither be created nor destroyed in the course of a Physical or chemical process although it may change from one form to another. Q4. Which of the following statement about a compound is incorrect?(L2) (I) A molecule of a compound has atom of different elements. (II) A compound canno ...
... Ans. Matter can neither be created nor destroyed in the course of a Physical or chemical process although it may change from one form to another. Q4. Which of the following statement about a compound is incorrect?(L2) (I) A molecule of a compound has atom of different elements. (II) A compound canno ...
UNIT 1. SOME BASIC CONCEPTS OF CHEMISTRY Concept
... Ans. Matter can neither be created nor destroyed in the course of a Physical or chemical process although it may change from one form to another. Q4. Which of the following statement about a compound is incorrect?(L2) (I) A molecule of a compound has atom of different elements. (II) A compound canno ...
... Ans. Matter can neither be created nor destroyed in the course of a Physical or chemical process although it may change from one form to another. Q4. Which of the following statement about a compound is incorrect?(L2) (I) A molecule of a compound has atom of different elements. (II) A compound canno ...
Multiple Choice
... ::S=Se:=S:: has zero formal charge. There are 4 bonds and 5 pairs of nonbonding electrons. The 2p electron is in an excited state, otherwise it would go into 2s. The 2 2s electrons and 3 2p electrons are valence (highest energy level) five. First ionized electron is from 1s. It takes the most ener ...
... ::S=Se:=S:: has zero formal charge. There are 4 bonds and 5 pairs of nonbonding electrons. The 2p electron is in an excited state, otherwise it would go into 2s. The 2 2s electrons and 3 2p electrons are valence (highest energy level) five. First ionized electron is from 1s. It takes the most ener ...
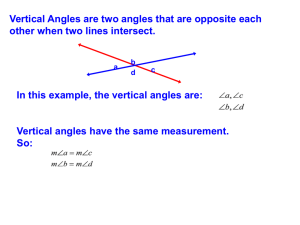
Adjacent, Vertical, Supplementary, and
... Corresponding Angles When two parallel lines are cut by a transversal, pairs of corresponding angles are formed. L Line L ...
... Corresponding Angles When two parallel lines are cut by a transversal, pairs of corresponding angles are formed. L Line L ...
Chem Agenda+ETDsHWK to End of Year 102714 Update
... Day 3: Atomic Theory Dalton-Thomson-Rutherford: BBs and Marbles Build your own super model Target: I can explain how to find the subatomic particle composition of any atom or isotope. A. Give the subatomic particle composition for Li, Mg, and F B. Why does atomic mass of C = 12.011? B. Reading the b ...
... Day 3: Atomic Theory Dalton-Thomson-Rutherford: BBs and Marbles Build your own super model Target: I can explain how to find the subatomic particle composition of any atom or isotope. A. Give the subatomic particle composition for Li, Mg, and F B. Why does atomic mass of C = 12.011? B. Reading the b ...
Exploring Angle Pairs
... Students will be able to: identify special angle pairs and use their relationships to find angle measures. ...
... Students will be able to: identify special angle pairs and use their relationships to find angle measures. ...
AP Book 8.1 sample
... anchors at the opposite sides of the pit in points W, X, Y, and Z. a) Draw the quadrilateral WXYZ. ...
... anchors at the opposite sides of the pit in points W, X, Y, and Z. a) Draw the quadrilateral WXYZ. ...
Angles
... Pairs Of Angles Formed by a Transversal A line that intersects two or more lines at different points is called a transversal. ...
... Pairs Of Angles Formed by a Transversal A line that intersects two or more lines at different points is called a transversal. ...
GEOMETRY: ANGLES
... “touch”. Linear Pair- adjacent angles that are also supplementary (their measurements add up to ...
... “touch”. Linear Pair- adjacent angles that are also supplementary (their measurements add up to ...
Chemistry Exemplar Problems
... development of syllabi and textbooks for all stages of school education. In this phase, a conscious effort has been made to discourage rote learning and to enhance comprehension. This is well in tune with the NPE-1986 and Learning Without Burden-1993 that recommend child centred system of education. ...
... development of syllabi and textbooks for all stages of school education. In this phase, a conscious effort has been made to discourage rote learning and to enhance comprehension. This is well in tune with the NPE-1986 and Learning Without Burden-1993 that recommend child centred system of education. ...
(Geometry) Lines and Angles
... Two polygons are similar if their corresponding sides are proportional. Similar Figures have the same shape, but not necessarily the same size. For Example: The triangles below are similar. Therefore, the measures of their corresponding angles are equal, and the corresponding sides are in proportion ...
... Two polygons are similar if their corresponding sides are proportional. Similar Figures have the same shape, but not necessarily the same size. For Example: The triangles below are similar. Therefore, the measures of their corresponding angles are equal, and the corresponding sides are in proportion ...
File
... Germanium is a relatively rare element and is classified as a semimetal. Its main uses are in the manufacture of semiconductors. Tin is a metal and is used to form alloys with other metals. Some alloys containing tin are bronze, solder, and pewter. Tin’s major current use is as a protective coating ...
... Germanium is a relatively rare element and is classified as a semimetal. Its main uses are in the manufacture of semiconductors. Tin is a metal and is used to form alloys with other metals. Some alloys containing tin are bronze, solder, and pewter. Tin’s major current use is as a protective coating ...
KCET – CHEMISTRY – 2016 - Medicine.careers360.com
... Which of the following is not true? 1) In vulcanization the rubber becomes harder and stronger 2) Natural rubber has ‘trans’ configuration at every double bond 3) Buna-S a co- polymer of Butene and styrene 4) Natural rubber is 1, 4 – polymer of isoprene ...
... Which of the following is not true? 1) In vulcanization the rubber becomes harder and stronger 2) Natural rubber has ‘trans’ configuration at every double bond 3) Buna-S a co- polymer of Butene and styrene 4) Natural rubber is 1, 4 – polymer of isoprene ...
Chapter 3: Ionic and Covalent Compounds Chapter 3: Ionic and
... 29. An atom has the electron-dot symbol shown below. If this atom is a main group element, what charge will it form in its ionic state? ...
... 29. An atom has the electron-dot symbol shown below. If this atom is a main group element, what charge will it form in its ionic state? ...
Section 2-5: Proving Angles Congruent
... • Complementary and supplementary angles do not have to be adjacent angles. • Complementary will always be only 2 angles whose sum is 90. • Supplementary must always be 2 angles whose sum is 180. ...
... • Complementary and supplementary angles do not have to be adjacent angles. • Complementary will always be only 2 angles whose sum is 90. • Supplementary must always be 2 angles whose sum is 180. ...
Bent's rule

Bent's rule describes and explains the relationship between the isovalent hybridization of central atoms in molecules and the electronegativities of substituents. The rule was stated by Henry Bent as follows: ""Atomic s character concentrates in orbitals directed toward electropositive substituents"".The chemical structure of a molecule is intimately related to its properties and reactivity. Valence bond theory proposes that molecular structures are due to covalent bonds between the atoms and that each bond consists of two overlapping and typically hybridised atomic orbitals. Traditionally, p-block elements in molecules are assumed to hybridise strictly as spn, where n is either 1, 2, or 3. In addition, the hybrid orbitals are all assumed to be equivalent (i.e. the n+1 spn orbitals have the same p character). Results from this approach are usually good, but they can be improved upon by allowing hybridised orbitals with noninteger and unequal p character. Bent's rule provides a qualitative estimate as to how these hybridised orbitals should be constructed. Bent's rule is that in a molecule, a central atom bonded to multiple groups will hybridise so that orbitals with more s character are directed towards electropositive groups, while orbitals with more p character will be directed towards groups that are more electronegative. By removing the assumption that all hybrid orbitals are equivalent spn orbitals, better predictions and explanations of properties such as molecular geometry and bond strength can be obtained.Bent's rule can be generalized to d-block elements as well. The hybridisation of a metal center is arranged so that orbitals with more s character are directed towards ligands that form bonds with more covalent character. Equivalently, orbitals with more d character are directed towards groups that form bonds of greater ionic character.