* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Biochemistry wikipedia , lookup
Nuclear chemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Analytical chemistry wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Marcus theory wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Chemical reaction wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Process chemistry wikipedia , lookup
Green chemistry wikipedia , lookup
Molecular dynamics wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Stoichiometry wikipedia , lookup
Reaction progress kinetic analysis wikipedia , lookup
George S. Hammond wikipedia , lookup
Computational chemistry wikipedia , lookup
Click chemistry wikipedia , lookup
Rate equation wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
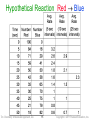
Chemistry: A Molecular Approach, 2nd Ed. Nivaldo Tro Chapter 13 Chemical Kinetics Roy Kennedy Massachusetts Bay Community College Wellesley Hills, MA Copyright 2011 Pearson Education, Inc. Ectotherms • Lizards, and other cold-blooded creatures, are • ectotherms – animals whose body temperature matches their environment’s temperature When a lizard’s body temperature drops, the chemical reactions that occur in its body slow down as do all chemical reactions when cooled • This causes the lizard to become lethargic and to • slow down Chemical kinetics is the study of the factors that affect the rates of chemical reactions Such as temperature Tro: Chemistry: A Molecular Approach, 2/e 2 Copyright 2011 Pearson Education, Inc. Chemical Kinetics • The speed of a chemical reaction is called its reaction rate • The rate of a reaction is a measure of how fast the reaction makes products or uses reactants • The ability to control the speed of a chemical reaction is important Tro: Chemistry: A Molecular Approach, 2/e 3 Copyright 2011 Pearson Education, Inc. Defining Rate • Rate is how much a quantity changes in a given period of time • The speed you drive your car is a rate – the distance your car travels (miles) in a given period of time (1 hour) so the rate of your car has units of mi/hr Tro: Chemistry: A Molecular Approach, 2/e 4 Copyright 2011 Pearson Education, Inc. Defining Reaction Rate • The rate of a chemical reaction is generally measured in terms of how much the concentration of a reactant decreases in a given period of time or product concentration increases • For reactants, a negative sign is placed in front of the definition Tro: Chemistry: A Molecular Approach, 2/e 5 Copyright 2011 Pearson Education, Inc. at t = 0 [A] = 8 [B] = 8 [C] = 0 at t = 0 [X] = 8 [Y] = 8 [Z] = 0 at t = 16 [A] = 4 [B] = 4 [C] = 4 at t = 16 [X] = 7 [Y] = 7 [Z] = 1 CA C2A2C1A 1 Rate t 2 t 2t1 t1 tt 44 0 8 .25 Rate 0.025 1616 0 0 Tro: Chemistry: A Molecular Approach, 2/e 6 1X 1 ZX Z2X2Z Rate Rate t 2 t 2t1t1 t t 708 1 Rate 0625 Rate 00..0625 116600 Copyright 2011 Pearson Education, Inc. at t = 16 [A] = 4 [B] = 4 [C] = 4 at t = 16 [X] = 7 [Y] = 7 [Z] = 1 at t = 32 [A] = 2 [B] = 2 [C] = 6 at t = 32 [X] = 6 [Y] = 6 [Z] = 2 CA C2AC 1A 1 2 Rate t 2 t12 t1 tt 62 44 Rate 0.125 0.125 3232 1616 Tro: Chemistry: A Molecular Approach, 2/e 7 ZX Z2XZ X1 1 2 Rate Rate t 2 t12 t1 tt 26 1 7 0.0625 Rate Rate 0.0625 3232 1616 Copyright 2011 Pearson Education, Inc. at t = 32 [A] = 2 [B] = 2 [C] = 6 at t = 32 [X] = 6 [Y] = 6 [Z] = 2 at t = 48 [A] = 0 [B] = 0 [C] = 8 at t = 48 [X] = 5 [Y] = 5 [Z] = 3 CA C2A2C1A 1 Rate t 2 tt21 t1 tt 80 6 2 .125 Rate 0.0125 48 3232 48 Tro: Chemistry: A Molecular Approach, 2/e 8 X2Z X1 ZX Z2 1 Rate Rate t 2 tt21 t1 tt 52 6 3 Rate .0625 Rate 0.00625 48 32 32 48 Copyright 2011 Pearson Education, Inc. Hypothetical Reaction Red Blue In this reaction, one molecule of Red turns into one molecule of Blue The number of molecules will always total 100 The rate of the reaction can be measured as the speed of loss of Red molecules over time, or the speed of gain of Blue molecules over time Tro: Chemistry: A Molecular Approach, 2/e 9 Copyright 2011 Pearson Education, Inc. Hypothetical Reaction Red Blue Tro: Chemistry: A Molecular Approach, 2/e 10 Copyright 2011 Pearson Education, Inc. Hypothetical Reaction Red Blue Tro: Chemistry: A Molecular Approach, 2/e 11 Copyright 2011 Pearson Education, Inc. Reaction Rate Changes Over Time • As time goes on, the rate of a reaction generally slows down because the concentration of the reactants decreases • At some time the reaction stops, either because the reactants run out or because the system has reached equilibrium Tro: Chemistry: A Molecular Approach, 2/e 12 Copyright 2011 Pearson Education, Inc. Reaction Rate and Stoichiometry • In most reactions, the coefficients of the balanced • equation are not all the same H2 (g) + I2 (g) 2 HI(g) For these reactions, the change in the number of molecules of one substance is a multiple of the change in the number of molecules of another for the above reaction, for every 1 mole of H2 used, 1 mole of I2 will also be used and 2 moles of HI made therefore the rate of change will be different • To be consistent, the change in the concentration of each substance is multiplied by 1/coefficient Tro: Chemistry: A Molecular Approach, 2/e 13 Copyright 2011 Pearson Education, Inc. Average Rate • The average rate is the change in measured concentrations in any particular time period linear approximation of a curve • The larger the time interval, the more the average rate deviates from the instantaneous rate Tro: Chemistry: A Molecular Approach, 2/e 14 Copyright 2011 Pearson Education, Inc. Hypothetical Reaction Red Blue Tro: Chemistry: A Molecular Approach, 2/e 15 Copyright 2011 Pearson Education, Inc. H2 I2 HI StoichiometryThe tellsaverage us that rate for is every 1 mole/L H2 used, theofchange in the 2 moles/L of concentration HI are made. in a time period Assuming a 1given L container, at 10 s, we used 0.181 moles of Inthe theamount first 10 s, H2. Therefore of the [H2] ismoles) −0.181=M, HI made is 2(0.181 0.362 moles.so the rate is At 60 s, we used 0.699 moles of H2. Therefore the amount of HI made is 2(0.699 moles) = 1.398 moles. Tro: Chemistry: A Molecular Approach, 2/e 16 Copyright 2011 Pearson Education, Inc. average rate in a given time period = slope of the line connecting the [H2] points; and ½ +slope of the line for [HI] the average rate for the first 80 s is 0.0108 M/s the average rate for the first 40 s is 0.0150 M/s the average rate for the first 10 s is 0.0181 M/s Tro: Chemistry: A Molecular Approach, 2/e 17 Copyright 2011 Pearson Education, Inc. Instantaneous Rate • The instantaneous rate is the change in concentration at any one particular time slope at one point of a curve • Determined by taking the slope of a line tangent to the curve at that particular point first derivative of the function for you calculus fans Tro: Chemistry: A Molecular Approach, 2/e 18 Copyright 2011 Pearson Education, Inc. H2 (g) + I2 (g) 2 HI (g) Using [H2], the instantaneous rate at 50 s is Using [HI], the instantaneous rate at 50 s is Tro: Chemistry: A Molecular Approach, 2/e 19 Copyright 2011 Pearson Education, Inc. Example 13.1: For the reaction given, the [I] changes from 1.000 M to 0.868 M in the first 10 s. Calculate the average rate in the first 10 s and the [H+]. H2O2 (aq) + 3 I(aq) + 2 H+(aq) I3(aq) + 2 H2O(l) solve the rate equation for the rate (in terms of the change in concentration of the given quantity) solve the rate equation (in terms of the change in the concentration for the quantity to find) for the unknown value Tro: Chemistry: A Molecular Approach, 2/e 20 Copyright 2011 Pearson Education, Inc. Practice – If 2.4 x 102 g of NOBr (MM 109.91 g) decomposes in a 2.0 x 102 mL flask in 5.0 minutes, find the average rate of Br2 production in M/s 2 NOBr(g) 2 NO(g) + Br2(l) Tro: Chemistry: A Molecular Approach, 2/e 21 Copyright 2011 Pearson Education, Inc. Practice – If 2.4 x 102 g of NOBr decomposes in a 2.0 x 102 mL flask in 5.0 minutes, find the average rate of Br2 production 2 NOBr(g) 2 NO(g) + Br2(l) 2 mL, Given: 240.0 5.45 MgBr NOBr, , 3.0 200.0 0.2000 x 10 sL, 5.0 5.0min. min. 2 Find: [Br2]/t, M/s Conceptual mole Br2 M Plan: Rate min s } Relationships: 2 mol NOBr:1 mol Br2, 1 mol = 109.91g, 1 mL=0.001 L Solve: Tro: Chemistry: A Molecular Approach, 2/e 22 Copyright 2011 Pearson Education, Inc. Measuring Reaction Rate • To measure the reaction rate you need to be able to measure the concentration of at least one component in the mixture at many points in time • There are two ways of approaching this problem 1. for reactions that are complete in less than 1 hour, it is best to use continuous monitoring of the concentration 2. for reactions that happen over a very long time, sampling of the mixture at various times can be used when sampling is used, often the reaction in the sample is stopped by a quenching technique Tro: Chemistry: A Molecular Approach, 2/e 23 Copyright 2011 Pearson Education, Inc. Continuous Monitoring • Polarimetry – measuring the change in the • degree of rotation of plane-polarized light caused by one of the components over time Spectrophotometry – measuring the amount of light of a particular wavelength absorbed by one component over time the component absorbs its complementary color • Total pressure – the total pressure of a gas mixture is stoichiometrically related to partial pressures of the gases in the reaction Tro: Chemistry: A Molecular Approach, 2/e 24 Copyright 2011 Pearson Education, Inc. Sampling the Reaction Mixture at Specific Times • At specific times during the reaction, drawing off aliquots, (samples from the reaction mixture), and doing quantitative analysis titration for one of the components gravimetric analysis • Gas chromatography can measure the concentrations of various components in a mixture for samples that have volatile components separates mixture by adherence to a surface Tro: Chemistry: A Molecular Approach, 2/e 25 Copyright 2011 Pearson Education, Inc. Methods for Determining Concentrations in a Mixture Tro: Chemistry: A Molecular Approach, 2/e 26 Copyright 2011 Pearson Education, Inc. Methods for Determining Concentrations in a Mixture Tro: Chemistry: A Molecular Approach, 2/e 27 Copyright 2011 Pearson Education, Inc. Methods for Determining Concentrations in a Mixture Tro: Chemistry: A Molecular Approach, 2/e 28 Copyright 2011 Pearson Education, Inc. Factors Affecting Reaction Rate: Nature of the Reactants • Nature of the reactants means what kind of reactant molecules and what physical condition they are in small molecules tend to react faster than large molecules gases tend to react faster than liquids, which react faster than solids powdered solids are more reactive than “blocks” more surface area for contact with other reactants certain types of chemicals are more reactive than others e.g. potassium metal is more reactive than sodium ions react faster than molecules no bonds need to be broken Tro: Chemistry: A Molecular Approach, 2/e 29 Copyright 2011 Pearson Education, Inc. Factors Affecting Reaction Rate: Temperature • Increasing temperature increases reaction rate chemist’s rule of thumb—for each 10 °C rise in temperature, the speed of the reaction doubles for many reactions • There is a mathematical relationship between the absolute temperature and the speed of a reaction discovered by Svante Arrhenius, which will be examined later Tro: Chemistry: A Molecular Approach, 2/e 30 Copyright 2011 Pearson Education, Inc. Factors Affecting Reaction Rate: Catalysts • Catalysts are substances that affect the speed • of a reaction without being consumed Most catalysts are used to speed up a reaction; these are called positive catalysts catalysts used to slow a reaction are called negative catalysts. • Homogeneous = present in same phase • Heterogeneous = present in different phase • How catalysts work will be examined later Tro: Chemistry: A Molecular Approach, 2/e 31 Copyright 2011 Pearson Education, Inc. Factors Affecting Reaction Rate: Reactant Concentration • Generally, the larger the concentration of reactant molecules, the faster the reaction increases the frequency of reactant molecule contact concentration of gases depends on the partial pressure of the gas higher pressure = higher concentration • Concentrations of solutions depend on the solute-to-solution ratio (molarity) Tro: Chemistry: A Molecular Approach, 2/e 32 Copyright 2011 Pearson Education, Inc. The Rate Law • The rate law of a reaction is the mathematical relationship between the rate of the reaction and the concentrations of the reactants and homogeneous catalysts as well • The rate law must be determined experimentally!! • The rate of a reaction is directly proportional to the concentration of each reactant raised to a power • For the reaction aA + bB products the rate law would have the form given below n and m are called the orders for each reactant k is called the rate constant Tro: Chemistry: A Molecular Approach, 2/e 33 Copyright 2011 Pearson Education, Inc. Reaction Order • The exponent on each reactant in the rate law • • is called the order with respect to that reactant The sum of the exponents on the reactants is called the order of the reaction The rate law for the reaction 2 NO(g) + O2(g) 2 NO2(g) is Rate = k[NO]2[O2] The reaction is second order with respect to [NO], first order with respect to [O2], and third order overall Tro: Chemistry: A Molecular Approach, 2/e 34 Copyright 2011 Pearson Education, Inc. Sample Rate Laws The bottom reaction is autocatalytic because a product affects the rate. Hg2+ is a negative catalyst; increasing its concentration slows the reaction. Tro: Chemistry: A Molecular Approach, 2/e 35 Copyright 2011 Pearson Education, Inc. Example: The rate equation for the reaction of NO with ozone is Rate = k[NO][O3]. If the rate is 6.60 x 10−5 M/sec when [NO] = 1.00 x 10−6 M and [O3] = 3.00 x 10−6 M, calculate the rate constant Given: Find: Conceptual Plan: Relationships: [NO] = 1.00 x 10−6 M, [O3] = 3.00 x 10−6 M, Rate = 6.60 x 10−6 M/s k, M−1s−1 Rate, [NO], [O3] k Rate = k[NO][O3] Solve: Tro: Chemistry: A Molecular Approach, 2/e 36 Copyright 2011 Pearson Education, Inc. Practice – The rate law for the decomposition of acetaldehyde is Rate = k[acetaldehyde]2. What is the rate of the reaction when the [acetaldehyde] = 1.75 x 10−3 M and the rate constant is 6.73 x 10−6 M−1•s−1? Tro: Chemistry: A Molecular Approach, 2/e 37 Copyright 2011 Pearson Education, Inc. Practice – What is the rate of the reaction when the [acetaldehyde] = 1.75 x 10−3 M and the rate constant is 6.73 x 10−6 M−1•s−1? Given: [acetaldehyde] = 1.75 x 10−3 M, k= 6.73 x 10−6 M−1•s−1 Find: Rate, M/s Conceptual k, [acetaldehyde] Rate Plan: Relationships: Rate = k[acetaldehyde]2 Solve: Tro: Chemistry: A Molecular Approach, 2/e 38 Copyright 2011 Pearson Education, Inc. Finding the Rate Law: the Initial Rate Method • The rate law must be determined experimentally • The rate law shows how the rate of a reaction • depends on the concentration of the reactants Changing the initial concentration of a reactant will therefore affect the initial rate of the reaction then doubling the initial concentration of A quadruples doubles does notthe the change initial reaction the initial rate reaction rate if for the reaction A → Products Tro: Chemistry: A Molecular Approach, 2/e 39 Copyright 2011 Pearson Education, Inc. Rate = k[A]n • If a reaction is Zero Order, the rate of the reaction is always the same doubling [A] will have no effect on the reaction rate • If a reaction is First Order, the rate is directly proportional to the reactant concentration doubling [A] will double the rate of the reaction • If a reaction is Second Order, the rate is directly proportional to the square of the reactant concentration doubling [A] will quadruple the rate of the reaction Tro: Chemistry: A Molecular Approach, 2/e 40 Copyright 2011 Pearson Education, Inc. Determining the Rate Law When there Are Multiple Reactants • Changing each reactant will effect the overall rate of the reaction • By changing the initial concentration of one reactant at a time, the effect of each reactant’s concentration on the rate can be determined • In examining results, we compare differences in rate for reactions that only differ in the concentration of one reactant Tro: Chemistry: A Molecular Approach, 2/e 41 Copyright 2011 Pearson Education, Inc. Example 13.2: Determine the rate law and rate constant for the reaction NO2(g) + CO(g) NO(g) + CO2(g) given the data below Comparing Expt #1 and Expt #2, the [NO2] changes but the [CO] does not Tro: Chemistry: A Molecular Approach, 2/e 42 Copyright 2011 Pearson Education, Inc. Example 13.2: Determine the rate law and rate constant for the reaction NO2(g) + CO(g) NO(g) + CO2(g) given the data below Tro: Chemistry: A Molecular Approach, 2/e 43 Copyright 2011 Pearson Education, Inc. Example 13.2: Determine the rate law and rate constant for the reaction NO2(g) + CO(g) NO(g) + CO2(g) given the data below Tro: Chemistry: A Molecular Approach, 2/e 44 Copyright 2011 Pearson Education, Inc. Example 13.2: Determine the rate law and rate constant for the reaction NO2(g) + CO(g) NO(g) + CO2(g) given the data below Tro: Chemistry: A Molecular Approach, 2/e 45 Copyright 2011 Pearson Education, Inc. Example 13.2: Determine the rate law and rate constant for the reaction NO2(g) + CO(g) NO(g) + CO2(g) given the data below n = 2, m = 0 Tro: Chemistry: A Molecular Approach, 2/e 46 Copyright 2011 Pearson Education, Inc. Example 13.2: Determine the rate law and rate constant for the reaction NO2(g) + CO(g) NO(g) + CO2(g) given the data below Substitute the concentrations and rate for any experiment into the rate law and solve for k Expt. Initial Initial Rate Initial Number [NO2], (M) [CO], (M) (M/s) 1. 2. 3. 0.10 0.20 0.20 0.10 0.10 0.20 0.0021 0.0082 0.0083 4. 0.40 0.10 0.033 Rate k[NO2 ]2 for expt 1 0.0021 M s k 0.10 M 2 0.0021 Ms 1 1 k 0.21 M s 0.01 M2 Tro: Chemistry: A Molecular Approach, 2/e 47 Copyright 2011 Pearson Education, Inc. Practice – Determine the rate law and rate constant for the reaction NH4+ + NO2− N2 + 2 H2O given the data below Tro: Chemistry: A Molecular Approach, 2/e 48 Copyright 2011 Pearson Education, Inc. Practice – Determine the rate law and rate constant for the reaction NH4+ + NO2− N2 + 2 H2O given the data below Rate = k[NH4+]n[NO2]m Tro: Chemistry: A Molecular Approach, 2/e 49 Copyright 2011 Pearson Education, Inc. Finding the Rate Law: Graphical Methods • The rate law must be determined experimentally • A graph of concentration of reactant vs. time can • • be used to determine the effect of concentration on the rate of a reaction This involves using calculus to determine the area under a curve – which is beyond the scope of this course Later we will examine the results of this analysis so we can use graphs to determine rate laws for several simple cases Tro: Chemistry: A Molecular Approach, 2/e 50 Copyright 2011 Pearson Education, Inc. Reactant Concentration vs. Time A Products insert figure 13.6 Tro: Chemistry: A Molecular Approach, 2/e 51 Copyright 2011 Pearson Education, Inc. Integrated Rate Laws • For the reaction A Products, the rate law • depends on the concentration of A Applying calculus to integrate the rate law gives another equation showing the relationship between the concentration of A and the time of the reaction – this is called the Integrated Rate Law Tro: Chemistry: A Molecular Approach, 2/e 52 Copyright 2011 Pearson Education, Inc. Half-Life • The half-life, t1/2, of a reaction is the length of time it takes for the concentration of the reactant to fall to ½ its initial value • The half-life of the reaction depends on the order of the reaction Tro: Chemistry: A Molecular Approach, 2/e 53 Copyright 2011 Pearson Education, Inc. Zero Order Reactions • Rate = k[A]0 = k constant rate reactions • [A] = −kt + [A]initial • Graph of [A] vs. time is straight line with • • slope = −k and y–intercept = [A]initial t ½ = [Ainitial]/2k When Rate = M/sec, k = M/sec [A]initial [A] time Tro: Chemistry: A Molecular Approach, 2/e 54 Copyright 2011 Pearson Education, Inc. First Order Reactions • Rate = k[A]1 = k[A] • ln[A] = −kt + ln[A]initial • Graph ln[A] vs. time gives straight line with slope = −k and y–intercept = ln[A]initial used to determine the rate constant • t½ = 0.693/k • The half-life of a first order reaction is constant • When Rate = M/sec, k = s–1 Tro: Chemistry: A Molecular Approach, 2/e 55 Copyright 2011 Pearson Education, Inc. ln[A]initial ln[A] time Tro: Chemistry: A Molecular Approach, 2/e 56 Copyright 2011 Pearson Education, Inc. The Half-Life of a First-Order Reaction Is Constant Tro: Chemistry: A Molecular Approach, 2/e 57 Copyright 2011 Pearson Education, Inc. Rate Data for C4H9Cl + H2O C4H9OH + HCl Tro: Chemistry: A Molecular Approach, 2/e 58 Copyright 2011 Pearson Education, Inc. C4H9Cl + H2O C4H9OH + 2 HCl Tro: Chemistry: A Molecular Approach, 2/e 59 Copyright 2011 Pearson Education, Inc. C4H9Cl + H2O C4H9OH + 2 HCl Tro: Chemistry: A Molecular Approach, 2/e 60 Copyright 2011 Pearson Education, Inc. C4H9Cl + H2O C4H9OH + 2 HCl slope = −2.01 x 10−3 k= 2.01 x 10−3 s-1 Tro: Chemistry: A Molecular Approach, 2/e 61 Copyright 2011 Pearson Education, Inc. Second Order Reactions • Rate = k[A]2 • 1/[A] = kt + 1/[A]initial • Graph 1/[A] vs. time gives straight line with slope = k and y–intercept = 1/[A]initial used to determine the rate constant • t½ = 1/(k[A0]) • When Rate = M/sec, k = M−1s−1 Tro: Chemistry: A Molecular Approach, 2/e 62 Copyright 2011 Pearson Education, Inc. 1/[A] 1/[A]initial time Tro: Chemistry: A Molecular Approach, 2/e 63 Copyright 2011 Pearson Education, Inc. Rate Data For 2 NO2 2 NO + O2 Tro: Chemistry: A Molecular Approach, 2/e 64 Copyright 2011 Pearson Education, Inc. Rate Data For 2 NO2 2 NO + O2 Tro: Chemistry: A Molecular Approach, 2/e 65 Copyright 2011 Pearson Education, Inc. Rate Data Graphs for 2 NO2 2 NO + O2 Tro: Chemistry: A Molecular Approach, 2/e 66 Copyright 2011 Pearson Education, Inc. Rate Data Graphs for 2 NO2 2 NO + O2 ln(PNO2) vs. Time 4.8 4.6 4.4 ln(pressure) 4.2 4 3.8 3.6 3.4 3.2 3 2.8 2.6 2.4 0 50 100 150 200 250 Time (hr) Tro: Chemistry: A Molecular Approach, 2/e 67 Copyright 2011 Pearson Education, Inc. Rate Data Graphs for 2 NO2 2 NO + O2 Tro: Chemistry: A Molecular Approach, 2/e 68 Copyright 2011 Pearson Education, Inc. Tro: Chemistry: A Molecular Approach, 2/e 69 Copyright 2011 Pearson Education, Inc. Example 13.4: The reaction SO2Cl2(g) SO2(g) + Cl2(g) is first order with a rate constant of 2.90 x 10−4 s−1 at a given set of conditions. Find the [SO2Cl2] at 865 s when [SO2Cl2]initial = 0.0225 M Given: [SO2Cl2]init = 0.0225 M, t = 865, k = 2.90 x 10-4 s−1 Find: [SO2Cl2] Conceptual Plan: [SO2Cl2]init, t, k [SO2Cl2] Relationships: Solution: Check: the new concentration is less than the original, as expected Tro: Chemistry: A Molecular Approach, 2/e 70 Copyright 2011 Pearson Education, Inc. Practice – The reaction Q 2 R is second order in Q. If the initial [Q] = 0.010 M and after 5.0 x 102 seconds the [Q] = 0.0010 M, find the rate constant Tro: Chemistry: A Molecular Approach, 2/e 71 Copyright 2011 Pearson Education, Inc. Practice – The reaction Q 2 R is second order in Q. If the initial [Q] = 0.010 M and after 5.0 x 102 seconds the [Q] = 0.0010 M, find the rate constant Given: [Q]init = 0.010 M, t = 5.0 x 102 s, [Q]t = 0.0010 M Find: k Conceptual Plan: [Q]init, t, [Q]t k Relationships: Solution: Tro: Chemistry: A Molecular Approach, 2/e 72 Copyright 2011 Pearson Education, Inc. Graphical Determination of the Rate Law for A Product • Plots of [A] vs. time, ln[A] vs. time, and 1/[A] vs. • time allow determination of whether a reaction is zero, first, or second order Whichever plot gives a straight line determines the order with respect to [A] if linear is [A] vs. time, Rate = k[A]0 if linear is ln[A] vs. time, Rate = k[A]1 if linear is 1/[A] vs. time, Rate = k[A]2 Tro: Chemistry: A Molecular Approach, 2/e 73 Copyright 2011 Pearson Education, Inc. Practice – Complete the Table and Determine the Rate Equation for the Reaction A 2 Product What will the rate be when the [A] = 0.010 M? Tro: Chemistry: A Molecular Approach, 2/e 74 Copyright 2011 Pearson Education, Inc. Practice – Complete the Table and Determine the Rate Equation for the Reaction A 2 Product Tro: Chemistry: A Molecular Approach, 2/e 75 Copyright 2011 Pearson Education, Inc. Tro: Chemistry: A Molecular Approach, 2/e 76 Copyright 2011 Pearson Education, Inc. Tro: Chemistry: A Molecular Approach, 2/e 77 Copyright 2011 Pearson Education, Inc. Tro: Chemistry: A Molecular Approach, 2/e 78 Copyright 2011 Pearson Education, Inc. Practice – Complete the table and determine the rate equation for the reaction A 2 Product Because the graph 1/[A] vs. time is linear, the reaction is second order, Rate k[A]2 k slope of the line 0.10 M–1 s –1 Tro: Chemistry: A Molecular Approach, 2/e 79 Copyright 2011 Pearson Education, Inc. Relationship Between Order and Half-Life • For a zero order reaction, the lower the initial concentration of the reactants, the shorter the half-life t1/2 = [A]init/2k • For a first order reaction, the half-life is independent of the concentration t1/2 = ln(2)/k • For a second order reaction, the half-life is inversely proportional to the initial concentration – increasing the initial concentration shortens the half-life t1/2 = 1/(k[A]init) Tro: Chemistry: A Molecular Approach, 2/e 80 Copyright 2011 Pearson Education, Inc. Example 13.6: Molecular iodine dissociates at 625 K with a firstorder rate constant of 0.271 s−1. What is the half-life of this reaction? Given: k = 0.271 s−1 Find: t1/2 Conceptual Plan: k t1/2 Relationships: Solution: Check: the new concentration is less than the original, as expected Tro: Chemistry: A Molecular Approach, 2/e 81 Copyright 2011 Pearson Education, Inc. Practice – The reaction Q 2 R is second order in Q. If the initial [Q] = 0.010 M and the rate constant is 1.8 M−1•s−1 find the length of time for [Q] = ½[Q]init Tro: Chemistry: A Molecular Approach, 2/e 82 Copyright 2011 Pearson Education, Inc. Practice – The reaction Q 2 R is second order in Q. If the initial [Q] = 0.010 M and the rate constant is 1.8 M−1•s−1 find the length of time for [Q] = ½[Q]init Given: [Q]init = 0.010 M, k = 1.8 M−1s−1 Find: t1/2, s Conceptual Plan: [Q]init, k t1/2 Relationships: Solution: Tro: Chemistry: A Molecular Approach, 2/e 83 Copyright 2011 Pearson Education, Inc. The Effect of Temperature on Rate • Changing the temperature changes the rate • constant of the rate law Svante Arrhenius investigated this relationship and showed that: where T is the temperature in kelvins R is the gas constant in energy units, 8.314 J/(mol•K) A is called the frequency factor, the rate the reactant energy approaches the activation energy Ea is the activation energy, the extra energy needed to start the molecules reacting Tro: Chemistry: A Molecular Approach, 2/e 84 Copyright 2011 Pearson Education, Inc. Tro: Chemistry: A Molecular Approach, 2/e 85 Copyright 2011 Pearson Education, Inc. Activation Energy and the Activated Complex • There is an energy barrier to almost all • reactions The activation energy is the amount of energy needed to convert reactants into the activated complex aka transition state • The activated complex is a chemical species with partially broken and partially formed bonds always very high in energy because of its partial bonds Tro: Chemistry: A Molecular Approach, 2/e 86 Copyright 2011 Pearson Education, Inc. Isomerization of Methyl Isonitrile methyl isonitrile rearranges to acetonitrile for the reaction to occur, the H3C─N bond must break, and a new H3C─C bond form Tro: Chemistry: A Molecular Approach, 2/e 87 Copyright 2011 Pearson Education, Inc. Energy Profile for the Isomerization of Methyl Isonitrile As the reaction begins, the the activated frequency activation energy is C─N the weakens number is complex the bond difference ofismolecules a in enough for the the that begin energy chemical between species to form the CNcomplex group activated reactants with partial and bonds thetoin start to rotate a given activated period complex of time Tro: Chemistry: A Molecular Approach, 2/e 88 Copyright 2011 Pearson Education, Inc. The Arrhenius Equation: The Exponential Factor • The exponential factor in the Arrhenius equation is a number between 0 and 1 • Tt represents the fraction of reactant molecules with sufficient energy so they can make it over the energy barrier the higher the energy barrier (larger activation energy), the fewer molecules that have sufficient energy to overcome it • That extra energy comes from converting the kinetic energy of motion to potential energy in the molecule when the molecules collide increasing the temperature increases the average kinetic energy of the molecules therefore, increasing the temperature will increase the number of molecules with sufficient energy to overcome the energy barrier therefore increasing the temperature will increase the reaction rate Tro: Chemistry: A Molecular Approach, 2/e 89 Copyright 2011 Pearson Education, Inc. Tro: Chemistry: A Molecular Approach, 2/e 90 Copyright 2011 Pearson Education, Inc. Arrhenius Plots • The Arrhenius Equation can be algebraically solved to give the following form: this equation is in the form y = mx + b where y = ln(k) and x = (1/T) a graph of ln(k) vs. (1/T) is a straight line (−8.314 J/mol∙K)(slope of the line) = Ea, (in Joules) ey–intercept = A (unit is the same as k) Tro: Chemistry: A Molecular Approach, 2/e 91 Copyright 2011 Pearson Education, Inc. Example 13.7: Determine the activation energy and frequency factor for the reaction O3(g) O2(g) + O(g) given the following data: Tro: Chemistry: A Molecular Approach, 2/e 92 Copyright 2011 Pearson Education, Inc. Example 13.7: Determine the activation energy and frequency factor for the reaction O3(g) O2(g) + O(g) given the following data: Tro: Chemistry: A Molecular Approach, 2/e 93 Copyright 2011 Pearson Education, Inc. Example 13.7: Determine the activation energy and frequency factor for the reaction O3(g) O2(g) + O(g) given the following data: slope, m = 1.12 x 104 K y–intercept, b = 26.8 Tro: Chemistry: A Molecular Approach, 2/e 94 Copyright 2011 Pearson Education, Inc. Arrhenius Equation: Two-Point Form • If you only have two (T,k) data points, the following forms of the Arrhenius Equation can be used: Tro: Chemistry: A Molecular Approach, 2/e 95 Copyright 2011 Pearson Education, Inc. Example 13.8: The reaction NO2(g) + CO(g) CO2(g) + NO(g) has a rate constant of 2.57 M−1∙s−1 at 701 K and 567 M−1∙s−1 at 895 K. Find the activation energy in kJ/mol Given: T1 = 701 K, k1 = 2.57 M−1∙s−1, T2 = 895 K, k2 = 567 M−1∙s−1 Find: Ea, kJ/mol Conceptual Plan: T1, k1, T2, k2 Ea Relationships: Solution: Check: most activation energies are tens to hundreds of kJ/mol – so the answer is reasonable Tro: Chemistry: A Molecular Approach, 2/e 96 Copyright 2011 Pearson Education, Inc. Practice – It is often said that the rate of a reaction doubles for every 10 °C rise in temperature. Calculate the activation energy for such a reaction. Hint: make and Tro: Chemistry: A Molecular Approach, 2/e T1 = 300 K, T2 = 310 K k2 = 2k1 97 Copyright 2011 Pearson Education, Inc. Practice – Find the activation energy in kJ/mol for increasing the temperature 10 ºC doubling the rate Given: T1 = 300 K, T1 = 310 K, k2 = 2 k1 Find: Ea, kJ/mol Conceptual Plan: T1, k1, T2, k2 Ea Relationships: Solution: Check: most activation energies are tens to hundreds of kJ/mol – so the answer is reasonable Tro: Chemistry: A Molecular Approach, 2/e 98 Copyright 2011 Pearson Education, Inc. Collision Theory of Kinetics • For most reactions, for a reaction to take place, the reacting molecules must collide with each other on average about 109 collisions per second • Once molecules collide they may react together or they may not, depending on two factors – 1. whether the collision has enough energy to "break the bonds holding reactant molecules together"; and 2. whether the reacting molecules collide in the proper orientation for new bonds to form Tro: Chemistry: A Molecular Approach, 2/e 99 Copyright 2011 Pearson Education, Inc. Effective Collisions Kinetic Energy Factor For a collision to lead to overcoming the energy barrier, the reacting molecules must have sufficient kinetic energy so that when they collide the activated complex can form Tro: Chemistry: A Molecular Approach, 2/e 100 Copyright 2011 Pearson Education, Inc. Effective Collisions Orientation Effect Tro: Chemistry: A Molecular Approach, 2/e 101 Copyright 2011 Pearson Education, Inc. Effective Collisions • Collisions in which these two conditions are • • met (and therefore lead to reaction) are called effective collisions The higher the frequency of effective collisions, the faster the reaction rate When two molecules have an effective collision, a temporary, high energy (unstable) chemical species is formed – the activated complex Tro: Chemistry: A Molecular Approach, 2/e 102 Copyright 2011 Pearson Education, Inc. Collision Theory and the Frequency Factor of the Arrhenius Equation • The Arrhenius Equation includes a term, A, • called the Frequency Factor The Frequency Factor can be broken into two terms that relate to the two factors that determine whether a collision will be effective Tro: Chemistry: A Molecular Approach, 2/e 103 Copyright 2011 Pearson Education, Inc. Collision Frequency • The collision frequency is the number of • collisions that happen per second The more collisions per second there are, the more collisions can be effective and lead to product formation Tro: Chemistry: A Molecular Approach, 2/e 104 Copyright 2011 Pearson Education, Inc. Orientation Factor • The orientation factor, p, is a statistical term • • • • relating the frequency factor to the collision frequency For most reactions, p < 1 Generally, the more complex the reactant molecules, the smaller the value of p For reactions involving atoms colliding, p ≈ 1 because of the spherical nature of the atoms Some reactions actually can have a p > 1 generally involve electron transfer Tro: Chemistry: A Molecular Approach, 2/e 105 Copyright 2011 Pearson Education, Inc. Orientation Factor • The proper orientation results when the atoms • are aligned in such a way that the old bonds can break and the new bonds can form The more complex the reactant molecules, the less frequently they will collide with the proper orientation reactions where symmetry results in multiple orientations leading to reaction have p slightly less than 1 • For most reactions, the orientation factor is less than 1 Tro: Chemistry: A Molecular Approach, 2/e 106 Copyright 2011 Pearson Education, Inc. Molecular Interpretation of Factors Affecting Rate – Reactant Nature • Reactions generally occur faster in solution than in pure substances mixing gives more particle contact particles separated, allowing more effective collisions per second forming some solutions breaks bonds that need to be broken • Some materials undergo similar reactions at different rates either because they have a (1) higher initial potential energy and are therefore closer in energy to the activated complex, or (2) because their reaction has a lower activation energy CH4 + Cl2 CH3Cl + HCl is about 12x faster than CD4 + Cl2 CD3Cl + DCl because the C─H bond is weaker and less stable than the C─D bond CH4 + X2 CH3X + HX occurs about 100x faster with F2 than with Cl2 because the activation energy for F2 is 5 kJ/mol but for Cl2 is 17 kJ/mol Tro: Chemistry: A Molecular Approach, 2/e 107 Copyright 2011 Pearson Education, Inc. Molecular Interpretation of Factors Affecting Rate – Temperature • Increasing the temperature raises the • • average kinetic energy of the reactant molecules There is a minimum amount of kinetic energy needed for the collision to be converted into enough potential energy to form the activated complex Increasing the temperature increases the number of molecules with sufficient kinetic energy to overcome the activation energy Tro: Chemistry: A Molecular Approach, 2/e 108 Copyright 2011 Pearson Education, Inc. Molecular Interpretation of Factors Affecting Rate – Concentration • Reaction rate generally increases as the concentration or partial pressure of reactant molecules increases except for zero order reactions • More molecules leads to more molecules with sufficient kinetic energy for effective collision distribution the same, just bigger curve Tro: Chemistry: A Molecular Approach, 2/e 109 Copyright 2011 Pearson Education, Inc. Reaction Mechanisms • We generally describe chemical reactions with an • • • • equation listing all the reactant molecules and product molecules But the probability of more than 3 molecules colliding at the same instant with the proper orientation and sufficient energy to overcome the energy barrier is negligible Most reactions occur in a series of small reactions involving 1, 2, or at most 3 molecules Describing the series of reactions that occurs to produce the overall observed reaction is called a reaction mechanism Knowing the rate law of the reaction helps us understand the sequence of reactions in the mechanism Tro: Chemistry: A Molecular Approach, 2/e 110 Copyright 2011 Pearson Education, Inc. An Example of a Reaction Mechanism • Overall reaction: • H2(g) + 2 ICl(g) 2 HCl(g) + I2(g) Mechanism: 1. H2(g) + ICl(g) HCl(g) + HI(g) 2. HI(g) + ICl(g) HCl(g) + I2(g) • The reactions in this mechanism are elementary steps, meaning that they cannot be broken down into simpler steps and that the molecules actually interact directly in this manner without any other steps Tro: Chemistry: A Molecular Approach, 2/e 111 Copyright 2011 Pearson Education, Inc. Elements of a Mechanism Intermediates H2(g) + 2 ICl(g) 2 HCl(g) + I2(g) 1) H2(g) + ICl(g) HCl(g) + HI(g) 2) HI(g) + ICl(g) HCl(g) + I2(g) • • • Notice that the HI is a product in Step 1, but then a reactant in Step 2 Because HI is made but then consumed, HI does not show up in the overall reaction Materials that are products in an early mechanism step, but then a reactant in a later step, are called intermediates Tro: Chemistry: A Molecular Approach, 2/e 112 Copyright 2011 Pearson Education, Inc. Molecularity • The number of reactant particles in an • • elementary step is called its molecularity A unimolecular step involves one particle A bimolecular step involves two particles though they may be the same kind of particle • A termolecular step involves three particles though these are exceedingly rare in elementary steps Tro: Chemistry: A Molecular Approach, 2/e 113 Copyright 2011 Pearson Education, Inc. Rate Laws for Elementary Steps • Each step in the mechanism is like its own • • little reaction – with its own activation energy and own rate law The rate law for an overall reaction must be determined experimentally But the rate law of an elementary step can be deduced from the equation of the step H2(g) + 2 ICl(g) 2 HCl(g) + I2(g) 1) H2(g) + ICl(g) HCl(g) + HI(g) Rate = k1[H2][ICl] 2) HI(g) + ICl(g) HCl(g) + I2(g) Tro: Chemistry: A Molecular Approach, 2/e 114 Rate = k2[HI][ICl] Copyright 2011 Pearson Education, Inc. Rate Laws of Elementary Steps Tro: Chemistry: A Molecular Approach, 2/e 115 Copyright 2011 Pearson Education, Inc. Rate Determining Step • In most mechanisms, one step occurs slower than the other steps • The result is that product production cannot occur any faster than the slowest step – the step determines the rate of the overall reaction • We call the slowest step in the mechanism the rate determining step the slowest step has the largest activation energy • The rate law of the rate determining step determines the rate law of the overall reaction Tro: Chemistry: A Molecular Approach, 2/e 116 Copyright 2011 Pearson Education, Inc. Another Reaction Mechanism NO2(g) + CO(g) NO(g) + CO2(g) 1. NO2(g) + NO2(g) NO3(g) + NO(g) 2. NO3(g) + CO(g) NO2(g) + CO2(g) Rateobs = k[NO2]2 Rate = k1[NO2]2 Slow Rate = k2[NO3][CO] Fast The first step is slower than the second step because its activation energy is larger. The first step in this mechanism is the rate determining step. The rate law of the first step is the same as the rate law of the overall reaction. Tro: Chemistry: A Molecular Approach, 2/e 117 Copyright 2011 Pearson Education, Inc. Validating a Mechanism • To validate (not prove) a mechanism, two conditions must be met: 1. The elementary steps must sum to the overall reaction 2. The rate law predicted by the mechanism must be consistent with the experimentally observed rate law Tro: Chemistry: A Molecular Approach, 2/e 118 Copyright 2011 Pearson Education, Inc. Mechanisms with a Fast Initial Step • When a mechanism contains a fast initial step, • the rate limiting step may contain intermediates When a previous step is rapid and reaches equilibrium, the forward and reverse reaction rates are equal – so the concentrations of reactants and products of the step are related and the product is an intermediate • Substituting into the rate law of the RDS will produce a rate law in terms of just reactants Tro: Chemistry: A Molecular Approach, 2/e 119 Copyright 2011 Pearson Education, Inc. An Example k1 1. 2 NO(g) N2O2(g) Fast 2. H2(g) + N2O2(g) H2O(g) + N2O(g) Slow Rate = k2[H2][N2O2] 3. H2(g) + N2O(g) H2O(g) + N2(g) Fast k−1 2 H2(g) + 2 NO(g) 2 H2O(g) + N2(g) Rateobs = k [H2][NO]2 Tro: Chemistry: A Molecular Approach, 2/e 120 Copyright 2011 Pearson Education, Inc. Example 13.9: Show that the proposed mechanism for the reaction 2 O3(g) 3 O2(g) matches the observed rate law Rate = k[O3]2[O2]−1 k1 1. O3(g) O2(g) + O(g) Fast k−1 2. O3(g) + O(g) 2 O2(g) Slow Tro: Chemistry: A Molecular Approach, 2/e 121 Rate = k2[O3][O] Copyright 2011 Pearson Education, Inc. Practice – Mechanism Determine the overall reaction, the rate determining step, the rate law, and identify all intermediates of the following mechanism 1. 2. A + B2 AB + B A + B AB Tro: Chemistry: A Molecular Approach, 2/e 122 Slow Fast Copyright 2011 Pearson Education, Inc. Practice – Mechanism Determine the overall reaction, the rate determining step, the rate law, and identify all intermediates of the following mechanism 1. 2. A + B2 AB + B A + B AB Slow Fast reaction 2 A + B2 2 AB B is an intermediate The first step is the rate determining step Rate = k[A][B2], same as RDS Tro: Chemistry: A Molecular Approach, 2/e 123 Copyright 2011 Pearson Education, Inc. Catalysts • Catalysts are substances that affect the rate of a • reaction without being consumed Catalysts work by providing an alternative mechanism for the reaction with a lower activation energy • Catalysts are consumed in an early mechanism step, then made in a later step mechanism without catalyst mechanism with catalyst O3(g) + O(g) 2 O2(g) V. Slow Cl(g) + O3(g) O2(g) + ClO(g) Fast ClO(g) + O(g) O2(g) + Cl(g) Slow Tro: Chemistry: A Molecular Approach, 2/e 124 Copyright 2011 Pearson Education, Inc. Ozone Depletion over the Antarctic Tro: Chemistry: A Molecular Approach, 2/e 125 Copyright 2011 Pearson Education, Inc. Molecular Interpretation of Factors Affecting Rate – Catalysts • Catalysts generally speed a reaction • They give the reactant molecules a different path to follow with a lower activation energy heterogeneous catalysts hold one reactant molecule in proper orientation for reaction to occur when the collision takes place and sometimes also help to start breaking bonds homogeneous catalysts react with one of the reactant molecules to form a more stable activated complex with a lower activation energy Tro: Chemistry: A Molecular Approach, 2/e 126 Copyright 2011 Pearson Education, Inc. Energy Profile of a Catalyzed Reaction polar stratospheric clouds contain ice crystals that catalyze reactions that release Cl from atmospheric chemicals Tro: Chemistry: A Molecular Approach, 2/e 127 Copyright 2011 Pearson Education, Inc. Practice – Mechanism Determine the overall reaction, the rate determining step, the rate law, and identify all catalysts and intermediates of the following mechanism 1. HQ2R2 + R− Q2R2− + HR Fast 2. Q2R2− Q2R + R− Slow Tro: Chemistry: A Molecular Approach, 2/e 128 Copyright 2011 Pearson Education, Inc. Practice – Mechanism Determine the overall reaction, the rate determining step, the rate law, and identify all catalysts and intermediates of the following mechanism 1. HQ2R2 + R− Q2R2− + HR Fast 2. Q2R2− Q2R + R− Slow reaction HQ2R2 Q2R + HR R− is a catalyst Q2R2− is an intermediate The second step is the rate determining step Rate = k[Q2R2−] = k[HQ2R2][R−] Tro: Chemistry: A Molecular Approach, 2/e 129 Copyright 2011 Pearson Education, Inc. Catalysts • Homogeneous catalysts are in the same phase as the reactant particles Cl(g) in the destruction of O3(g) • Heterogeneous catalysts are in a different phase than the reactant particles solid catalytic converter in a car’s exhaust system Tro: Chemistry: A Molecular Approach, 2/e 130 Copyright 2011 Pearson Education, Inc. Types of Catalysts Tro: Chemistry: A Molecular Approach, 2/e 131 Copyright 2011 Pearson Education, Inc. Catalytic Hydrogenation H2C=CH2 + H2 → CH3CH3 Tro: Chemistry: A Molecular Approach, 2/e 132 Copyright 2011 Pearson Education, Inc. Enzymes • Because many of the molecules are large and complex, most biological reactions require a catalyst to proceed at a reasonable rate • Protein molecules that catalyze biological reactions are called enzymes • Enzymes work by adsorbing the substrate reactant onto an active site that orients the substrate for reaction 1) Enzyme + Substrate Enzyme─Substrate Fast 2) Enzyme─Substrate → Enzyme + Product Tro: Chemistry: A Molecular Approach, 2/e 133 Slow Copyright 2011 Pearson Education, Inc. Enzyme–Substrate Binding: the Lock and Key Mechanism Tro: Chemistry: A Molecular Approach, 2/e 134 Copyright 2011 Pearson Education, Inc. Enzymatic Hydrolysis of Sucrose Tro: Chemistry: A Molecular Approach, 2/e 135 Copyright 2011 Pearson Education, Inc.