Critical issues in the heteroepitaxial growth of alkaline
... however, presents serious complications concerning reactivity between the desired oxide and silicon and delicate oxidation considerations. One demonstrated route that addresses the concerns of the transition from silicon to a more complex oxide 共specifically perovskite兲 is through an intermediate al ...
... however, presents serious complications concerning reactivity between the desired oxide and silicon and delicate oxidation considerations. One demonstrated route that addresses the concerns of the transition from silicon to a more complex oxide 共specifically perovskite兲 is through an intermediate al ...
Synthesis, identification and thermal decomposition of double
... usually oxides of the respective metals. Thus far, the effect of atmosphere by their heating has not been studied. The study of thermal behavior of mixed valence sul®te may help to understand the stability of the SIV compounds in the environment. Nevertheless, there are no data about thermal analysi ...
... usually oxides of the respective metals. Thus far, the effect of atmosphere by their heating has not been studied. The study of thermal behavior of mixed valence sul®te may help to understand the stability of the SIV compounds in the environment. Nevertheless, there are no data about thermal analysi ...
Studies on Veratryl Alcohol Oxidation Catalyzed by Co(salen) Type
... A complete four-electron reduction of molecular oxygen to produce two water molecules has a very high free energy change, but is restricted by mechanistic limitations. Usually the reduction of dioxygen occurs via successive steps of one- or two-electron transfer.35 Thus, the compounds, which can re ...
... A complete four-electron reduction of molecular oxygen to produce two water molecules has a very high free energy change, but is restricted by mechanistic limitations. Usually the reduction of dioxygen occurs via successive steps of one- or two-electron transfer.35 Thus, the compounds, which can re ...
pH and ORP Applications Cyanide Waste Treatment
... The ORP titration curve, Figure 2, shows the entire millivolt range if cyanide is treated as a batch. With continuous treatment, operation is maintained in the oxidized, positive region of the curve near the nominal +450 mV setpoint. The ORP setpoint can vary from installation to installation, depe ...
... The ORP titration curve, Figure 2, shows the entire millivolt range if cyanide is treated as a batch. With continuous treatment, operation is maintained in the oxidized, positive region of the curve near the nominal +450 mV setpoint. The ORP setpoint can vary from installation to installation, depe ...
The Covalent Potential: A Simple and Useful Measure of the
... where n, is the number of valence electrons in the bonding atom in X, where X represents an atom or group centered on X. In this way, our definition of EN differs from Yuan’s although our rx is the same as his. Note that the concept of group EN becomes unnecessary for our use. This again differs fro ...
... where n, is the number of valence electrons in the bonding atom in X, where X represents an atom or group centered on X. In this way, our definition of EN differs from Yuan’s although our rx is the same as his. Note that the concept of group EN becomes unnecessary for our use. This again differs fro ...
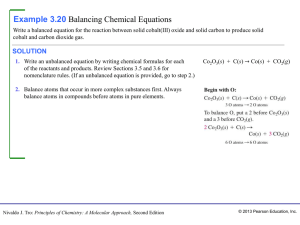
CLASSES AND NOMENCLATURE OF INORGANIC COMPOUNDS
... 15. The atomic number of chemical element is: A the number of protons in the nuclear of atom. B the number of neutrons in the nuclear of atom. C the number of nucleons in the nuclear of atom D the number of protons and neutrons in the nuclear of atom E the mass of atomic nuclear. A Ni B Cu C Zn D Pt ...
... 15. The atomic number of chemical element is: A the number of protons in the nuclear of atom. B the number of neutrons in the nuclear of atom. C the number of nucleons in the nuclear of atom D the number of protons and neutrons in the nuclear of atom E the mass of atomic nuclear. A Ni B Cu C Zn D Pt ...
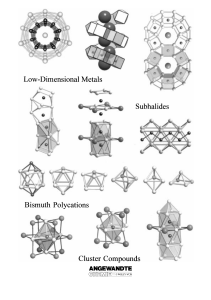
From the Metal to the Molecule
... significant progress has been made especially in research using the elements indium,[6] tin,[7] and bismuth.[8] This research mainly concerns the ternary and quaternary oxides or halides of the aforementioned main group elements which contain electron-rich transition metals from Groups 8 ± 10 as fur ...
... significant progress has been made especially in research using the elements indium,[6] tin,[7] and bismuth.[8] This research mainly concerns the ternary and quaternary oxides or halides of the aforementioned main group elements which contain electron-rich transition metals from Groups 8 ± 10 as fur ...
Local structure of LiNi0.5Mn0.5O2 cathode material probed by in
... photoelectron spectroscopy 共XPS兲 methods.21,22 It is important to note here that in contrast to XRD which gives one a good picture of the long-range structural changes in the cathode material, in situ XAS investigations during cycling are very illuminating in their own right, providing an understand ...
... photoelectron spectroscopy 共XPS兲 methods.21,22 It is important to note here that in contrast to XRD which gives one a good picture of the long-range structural changes in the cathode material, in situ XAS investigations during cycling are very illuminating in their own right, providing an understand ...