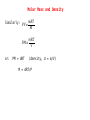* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Significant Figures
Gas chromatography wikipedia , lookup
Organic chemistry wikipedia , lookup
Process chemistry wikipedia , lookup
Transition state theory wikipedia , lookup
Computational chemistry wikipedia , lookup
Chemical bond wikipedia , lookup
Atomic nucleus wikipedia , lookup
Click chemistry wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Oxidation state wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Inductively coupled plasma mass spectrometry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Electrolysis of water wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
Chemical reaction wikipedia , lookup
Electrochemistry wikipedia , lookup
History of chemistry wikipedia , lookup
Biochemistry wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Thermometric titration wikipedia , lookup
Isotopic labeling wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Implicit solvation wikipedia , lookup
Extended periodic table wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
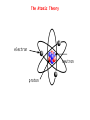
Significant Figures Zeros appearing between nonzero digits are significant, for example: * 60.8 has three significant figures * 39008 has five significant figures Zeros appearing in front of nonzero digits are not significant, for example: * 0.093827 has five significant figures * 0.0008 has one significant figure * 0.012 has two significant figures Significant Figures Zeros at the end of a number and to the right of a decimal are significant, for example: * 35.00 has four significant figures * 8,000.000000 has ten significant figures Zeros at the end of a number without a decimal point may or may not be significant, and are therefore ambiguous, for example: * 1,000 could have between one and four significant figures. This ambiguity could be resolved by placing a decimal after the number, e.g. writing "1,000." to indicate specifically that four significant figures are meant, but this is a non-standard usage. To specify unambiguously how many significant figures are implied, scientific notation can be employed: * 1×103 has one significant figure, while * 1.000×103 has four. Significant Figures Multiplication and Division When multiplying or dividing numbers, the result is rounded to the number of significant figures in the factor with the least significant figures. Here, the quantity of significant figures in each of the factors is important—not the position of the significant figures. For instance, using significance arithmetic rules: * * * * * * * 8 × 8 = 6 × 101 8 × 8.0 = 6 × 101 8.0 × 8.0 = 64 8.02 × 8.02 = 64.3 8 / 2.0 = 4 8.6 /2.0012 = 4.3 2 × 0.8 = 2 Significant Figures Addition and Subtraction When adding or subtracting using significant figures rules, results are rounded to the position of the least significant digit in the most uncertain of the numbers being summed (or subtracted). That is, the result is rounded to the last digit that is significant in each of the numbers being summed. Here the position of the significant figures is important, but the quantity of significant figures is irrelevant. Some examples using these rules: * 1 + 1.1 = 2 - 1 is significant up to the ones place, 1.1 is significant up to the tenths place. Of the two, the least accurate is the ones place. The answer cannot have any significant figures past the ones place. * 1.0 + 1.1 = 2.1 - 1.0, 1.1 are significant up to the tenths place. So will the answer. Significant Figures Addition and Subtraction * 100 + 110 = 210 - 100, 110 are significant up to the ones place, even though these digits are zeroes. So will the answer. * 1×102 + 1.1×102 = 2×102 - 100 is significant up to the hundreds place, while 110 is up to the tens place. Of the two, the least accurate is the hundreds place. The answer should not have significant digits past the hundreds place. Significant Figures Exact Numbers Some numbers are considered to have an infinite number of significant figures and are called exact numbers. An example would be a whole number from a balanced equation: 6.021 x 4/2 = 12.04 where 4/2 is perhaps the stoichiometric ratio of product to reactant. Since both 4 and 2 are exact numbers they can be considered to each have more significant figures than 6.021 which has only four significant figures. Thus, the rules for multiplication and division indicate that the result should have four significant figures. Significant Figures Both Multiplication/Division and Addition/Subtraction with Significant Figures ● when doing different kinds of operations with measurements with significant figures, do whatever is in parentheses first, evaluate the significant figures in the intermediate answer, then do the remaining steps 3.489 × (5.67 – 2.3) = 2 dp 1 dp 3.489 × 3.37 = 12 4 sf 1 dp & 2 sf 2 sf Significant Figures Calculations In a long calculation involving mixed operations, carry as many digits as possible through the entire set of calculations and then round the final result appropriately. For example, (5.00 / 1.235) + 3.000 + (6.35 / 4.0)=4.04858... + 3.000 + 1.5875=8.630829... The first division should result in 3 significant figures. The last division should result in 2 significant figures. The three numbers added together should result in a number that is rounded off to the last common significant digit occurring furthest to the right; in this case, the final result should be rounded with 1 digit after the decimal. Thus, the correct rounded final result should be 8.6. This final result has been limited by the accuracy in the last division. Significant Figures Example Problems 5.692 m + 0.6 m + 4.33 m = 10.6 m 4.51 cm x 3.6666 cm = 16.5 cm2 7.310 km / 5.70 km = 1.28 3.00 x 10-5 – 1.5 x 10-2 = 0.0000300 - 0.015 = -0.01497 = -1.5 x 102 Dimensional Analysis ● ● ● ● ● Many problems in chemistry involve using relationships to convert one unit of measurement to another Conversion factors are relationships between two units – May be exact or measured Conversion factors generated from equivalence statements 2.54cm 1 in – e.g., 1 inch = 2.54 cm can give or 1 in 2.54 cm Arrange conversion factors so given unit cancels – Arrange conversion factor so given unit is on the bottom of the conversion factor May string conversion factors – So we do not need to know every relationship, as long as we can find something else the given and desired units are related to Dimensional Analysis Example Problems 1 lb = 453.6 g convert 0.0833 lb to milligrams 0.0833 lb x 453.6 g/lb x 1000 mg/g = 3.78 x 105 mg 1 mi = 1609 m convert 11.0 x 103 km/s to mi/hr km m 1 mi s 11.0×10 ×1000 × ×3600 s km 1609m hr 3 =2.46×107 mi hr The Atomic Theory 1. Elements are composed of atoms. - tiny, hard, unbreakable spheres. 2. All atoms of a given element are identical - all carbon atoms have the same chemical and physical properties 3. Atoms of a given element are different from those of another element. - carbon atoms have different chemical and physical properties than sulfur atoms. 4. Atoms of one element can combine with atoms of another element to form compounds. - Law of Definite Proportions - Chemical formulas 5. Atoms are indivisible in chemical processes (Lavoisier). - all atoms present at the beginning of a chemical reaction are present at the end of the reaction - atoms of one element cannot change into atoms of another element The Atomic Theory electron neutron proton The Atomic Theory Isotopes ● ● ● ● ● all isotopes of an element are chemically identical – undergo the exact same chemical reactions all isotopes of an element have the same number of protons isotopes of an element have different masses isotopes of an element have different numbers of neutrons isotopes are identified by their mass numbers – protons + neutrons The Atomic Theory Isotopes • Atomic Number Number of protons example: Lithium – 3 protons Z -Z=3 • Mass Number Protons + Neutrons example: Nitrogen-15 15 7N Whole number • Abundance = relative amount found in a sample Atomic Mass Units Instead of using hydrogen, the lightest element, for historical reasons, we arbitrarily assign the carbon-12 atom a mass of exactly 12 atomic mass units (12 amu or simply 12 u) and then scale the mass of every other atom to that. Thus, atomic masses are actually relative masses. Note that while mass numbers are whole numbers representing the sum of protons and neutrons in the nucleus, atomic masses are not generally whole numbers. The Atomic Theory Isotopes The weighted average: Let's say that we have two sets of data with 20 in the first and 30 in the second: 1: 62, 67, 71, 74, 76, 77, 78, 79, 79, 80, 80, 81, 81, 82, 83, 84, 86, 89, 93, 98 2: 81, 82, 83, 84, 85, 86, 87, 87, 88, 88, 89, 89, 89, 90, 90, 90, 90, 91, 91, 91, 92, 92, 93, 93, 94, 95, 96, 97, 98, 99 average 1: 80 average 2: 90 What is the overall average? The Atomic Theory Isotopes Two ways to calculate overall average. 1. sum all numbers and divide by total: 4600/50 = 86 2. calculate a weighted average: 20×8030×90 =86 2030 or 20 30 ×80 ×90 2030 2030 The Atomic Theory Isotopes The average atomic mass is a weighted average of atomic masses of isotopes. avg. mass= fraction A×mass A fraction B×mass B The Atomic Theory Isotopes Example Problems How many protons, neutrons and electrons in: a) 17 8 b) 199 80 c) 63 29 O 8 protons, 8 electrons, 9 neutrons Hg 80 protons, 80 electrons, 119 neutrons Cu 2+ 29 protons, 27 electrons, 34 neutrons The Atomic Theory Isotopes Example Problems Potassium has two major isotopes, potassium-39 and potassium-41. The atomic mass of potassium-39 is 38.964 amu and its percent abundance is 93.1%. If the average atomic mass of potassium is 39.098 amu what is the atomic mass of potassium-41? 41 39.098=0.931×38.9640.069×Mass K 41 Mass K = 39.098−0.931×38.964 0.069 = 41 Chemical Compounds Molar Mass The mass of 1 mol of a substance in grams is defined as it’s molar mass. Since a mol of a substance is an Avogadro’s number of particles we can simply use the numerical value of the atomic mass of the element from the periodic table in grams units to find the molar mass. Thus: The molar mass of H atoms is 1.008 g/mol The molar mass of H2 molecules is 2.016 g/mol The molar mass of H2O molecules is 2 X 1.008 + 16.00 = 18.02 g/mol Thus, for compounds we can find the molar mass (or the formula mass) by using the molecular formula or the formula unit and simply adding up the atomic masses of each atom in the formula. The Mole We generally use the SI counting unit, the mole, to represent an Avogadro’s number of atoms or molecules. The numerical value of the mole is defined as being equal to the number of atoms in exactly 12 grams of pure carbon-12. This is an Avogadro's number of carbon-12 atoms or 6.022 x 1023 atoms. The mole is nothing more than a counting unit like “dozen” or “pair”. It's definition is purely for convenience to make the atomic masses of the elements in atomic mass units (which are hard to weigh in the lab) coincide with molar masses of the elements. Chemical Compounds Calculate the molar mass of V2O4 and Na2CO3. One mole of V2O4 contains two mols of V and four mols of O and the masses of these would be: 2 x 50.942 g/mol + 4 x 15.999 g/mol = 165.88 g/mol One mole of Na2CO3 contains two mols of Na, one mol of C and three mols of O. The molar mass is: 2 x 22.990 g/mol + 12.011 g/mol + 3 x 15.999 g/mol = 105.99 g/mol The Mole How many moles of ions are in 27.5 g of MgCl2? MgCl2 Mg2+ + 2Cl- mols MgCl2 = 27.5 g / 95.21 g/mol = 0.289 mol mols ions = 3 x mols MgCl2 = 3 x 0.289 mol = 0.867 mol The Mole What is the mass in grams of 7.70 x 1020 molecules of caffeine, C8H10N4O2? Mass of one mol of caffeine = 194.19 g/mol This is the mass of an Avogadro's number of caffeine molecules, 6.022 x 1023. mass of 7.70 x 1020 molecules of caffeine = 20 7.70×10 molecules ×194.19g /mol 23 6.022×10 molecules/mol =0.248 g The Mole A sample of glucose, C6H12O6, contains 1.25 x 1021 atoms of carbon. How many atoms of hydrogen does it contain? How many mols of glucose does it contain? From the formula for glucose there are twice as many hydrogens as carbons so there must be 2 x 1.25 x 1021 = 2.50 x 1021 atoms of hydrogen. Since each molecule has 6 carbons the number of glucose molecules will be 1.25 x 1021 / 6 = 2.08 x 1020. Division by Avogadro's number will give the number of mols of glucose: 20 2.08×10 molecules −4 =3.46×10 mol 23 6.022×10 molecules/mol Chemical Compounds Empirical Formula Summary of empirical formula determination steps: 1. Use the given % composition, or determine what the %composition is and then find the mass of each element in a 100 g sample of the substance. 2. For each elemental mass, determine the number of mols of the element in the 100 g sample using the corresponding atomic mass from the periodic table. 3. If necessary, divide each mol quantity by the smallest of the numbers of mols to get whole numbers. If any fractions result, multiply all results by the appropriate number to obtain all whole numbers. 4. Use the whole numbers from step 3 to generate an empirical formula. Chemical Compounds Empirical Formula When a 0.350 g sample of cinnabar containing Hg and S is heated it decomposes to give 0.302 g Hg. What is the empirical formula of cinnabar? Mols Hg = 0.302 g / 200.59 g/mol = 1.51 x 10-3 mol mass S = 0.350 g – 0.302 g = 0.048 g mols S = 0.048 g / 32.065 g/mol = 1.5 x 10-3 mols mol ratio Hg:S 1.51 x 10-3:1.5 x 10-3 or 1:1 empirical formula HgS Chemical Compounds Empirical Formula A sample of a chromium compound has a molar mass of 151.99 g/mol. Elemental analysis shows that it is composed of 68.43% Cr and 31.57% O. What are the empirical and molecular formulas for this compound? In a hypothetical 100 g sample there will be 68.43 g Cr and 31.57 g O. mols Cr = 68.43 g / 51.996 g/mol = 1.316 mol mols O = 31.57 g / 15 999 g/mol = 1.973 mol mol ratio V:O 1.316:1.973 or 1:1.5 or 2:3 empirical formula: V2O3 empirical formula mass: 149.88 g/mol ratio molar mass to efm 151.99:149.88 or 1:1 molecular formula is V2O3 Oxidation States Rules for assigning oxidation numbers: 1. The oxidation state of an atom in it's elemental form is zero. 2. For ions composed of only one atom the oxidation number is equal to the charge on the ion. 3. The oxidation state of oxygen in most compounds is -2. A notable exception is in peroxides such as hydrogen peroxide in which the oxidation state of oxygen is -1. 4. The oxidation state of hydrogen is generally +1 except when it is bonded to metals such as sodium (NaH) in which case it's oxidation number is -1. Oxidation States 5. Fluorine has an oxidation number of -1 in its compounds ... always. 6. The sum of the oxidation numbers of all of the atoms in a molecule or ion must equal the net charge on the molecule or ion. 7. Oxidation numbers do not necessarily have to be integers. ie. In Fe3O4, iron has an apparent oxidation number of +2 2/3. Oxidation States What is the oxidation number of V in V2O4? 4 x O = -8 2xV= ? ----Total charge 0 Oxidation number on each V must be +4 Nomenclature of Compounds Metal-containing Binary Compounds Compounds containing only two different elements are binary compounds and are named by using the element name of the first element followed by the name of the second element modified with the suffix 'ide'. exceptions: NaCl Al2O3 sodium chloride aluminum oxide NaOH KCN sodium hydroxide potassium cyanide Nomenclature of Compounds Many metals can exist in more than one oxidation state and form can form two or more compounds with a given anion. Fe+2 Fe+3 ferrous ion ferric ion FeCl2 FeCl3 ferrous chloride or iron(II) chloride ferric chloride or iron(III) chloride Binary Acids When binary compounds with acidic properties are discussed in the context of their acidity we alter their names by using the prefix 'hydro' and suffix 'ic': HCl HF HCN hydrochloric acid hydrofluoric acid hydrocyanic acid Nomenclature of Compounds Most polyatomic ions are anions rather than cations and the most common modifiers used as suffixes are 'ate' and 'ite', depending primarily on oxidation numbers. Also, it is most common for polyatomic anions to have oxygen in them and are called oxoanions. NH4+ CO3-2 NO3NO2- ammonium carbonate nitrate nitrite The 'ate' suffix is applied to the anion which has the primary atom in the higher oxidation state and 'ite' to the anion with the primary atom in the lower oxidation state. See Table 3.5 in the text for an extensive list of anions and their names. You will need to know these! Nomenclature of Compounds Oxo Acids (acids of polyatomic anions) H2CO3 H3BO3 carbonic acid boric acid H3PO4 H3PO3 phosphoric acid phosphorous acid H2SO4 H2SO3 sulfuric acid sulfurous acid HClO HClO2 HClO3 HClO4 hypochlorous acid chlorous acid chloric acid perchloric acid Nomenclature of Compounds Binary Compounds of Nonmetals The nomenclature of these compounds is similar to the binary compounds mentioned earlier except for those in which the primary element can exist in multiple oxidation states. HCl hydrogen chloride PCl5 PCl3 CO2 CO P4O6 phosphorous pentachloride phosphorous trichloride carbon dioxide carbon monoxide tetraphosphorus hexaoxide Nomenclature of Compounds Name these compounds: NiO, N2O5, HgCl2 Nickel (II) oxide Dinitrogen pentoxide Mercury (II) chloride Stoichiometry Chlorine dioxide is a bleaching agent used in the paper industry and can be prepared by the following reaction: NaClO2 + Cl2 ClO2 + NaCl What mass of Cl2 is needed for complete conversion of 30.5 g of NaClO2 ? Balance the equation: 2NaClO2 + Cl2 2ClO2 + 2NaCl Mols NaClO2 = 30.5 g / 90.442 g/mol = 0.337 mol mols Cl2 = 0.337 mol NaClO2 x 1 mol Cl2 / 2 mol NaClO2 = 0.169 mol Stoichiometry Chlorine dioxide is a bleaching agent used in the paper industry and can be prepared by the following reaction: NaClO2 + Cl2 ClO2 + NaCl What mass of Cl2 is needed for complete conversion of 30.5 g of NaClO2 ? Mass Cl2 = 0.169 mol x 70.91 g/mol = 12.0 g Stoichiometry The fertilizer ammonium sulfate is prepared by the reaction between ammonia and sulfuric acid: 2NH3 + H2SO4 (NH4)2SO4 + 2H2O What mass of NH3 in kg is needed to make 3.5 metric tons of ammonium sulfate? Mols (NH4)2SO4 = 3.5 tons x 1000 kg/ton x 1000 g/kg / 132.14 g/mol = 2.6 x 104 mols mols NH3 = 2.6 x 104 mols (NH4)2SO4 x 1 mol NH3/ 1 mol (NH4)2SO4 = 2.6 x 104 mols mass NH3 = 2.6 x 104 x 17.03 g/mol = 4.5 x 105g or 4.5 x 102 kg Limiting Reagents For the reaction between Zn and HCl: Zn + 2HCl H2 + ZnCl2 if 12.5 g HCl and 7.3 g Zn are reacted how much ZnCl2 can be expected? Assume HCl is limiting: mols HCl = 12.5 g / 36.46 g/mol = 0.343 mol mols ZnCl2 = 0.343 mol HCl x 1 mol ZnCl2 / 2 mol HCl = 0.171 mol Limiting Reagents For the reaction between Zn and HCl: Zn + 2HCl H2 + ZnCl2 if 12.5 g HCl and 7.3 g Zn are reacted how much ZnCl2 can be expected (in grams)? Assume Zn is limiting: mols Zn = 7.3 g / 65.409 g/mol = 0.11 mol mols ZnCl2 = 0.11 mol Zn x 1 mol ZnCl2 / 1 mol Zn = 0.11 mol This is the smaller of the two so Zn is the limiting reagent. Limiting Reagents For the reaction between Zn and HCl: Zn + 2HCl H2 + ZnCl2 if 12.5 g HCl and 7.3 g Zn are reacted how much ZnCl2 can be expected (in grams)? Mass ZnCl2 = 0.11 mol x 136.296 g/mol = 15 g Reaction Yield The theoretical yield of a reaction is the maximum amount of product that can be obtained for a given amount of limiting reagent. In practice, the theoretical yield is rarely obtained ... most often the actual yield is less than the theoretical yield. The efficiency of the reaction is described by the %yield: % yield = (actual yield/theoretical yield) x 100 Reaction Yield Determine the % yield for the reaction: KClO3 KCl + O2 if 2.41 g KClO3 produces 0.67 g O2. 2KClO3 2KCl + 3O2 First, calculate the theoretical yield: mols KClO3 = 2.41 g / 122.55 g/mol = 0.0197 mol mols O2 = 0.0197 mol KClO3 x 3 mol O2 / 2 mol KClO3 = 0.0295 mol mass O2 = 0.0295 x 31.999 g/mol = 0.944 g Reaction Yield Determine the % yield for the reaction: KClO3 KCl + O2 if 2.41 g KClO3 produces 0.67 g O2. Now the percent yield: % yield = (0.67 g/ 0.944 g) x 100 = 71 % Solution Stoichometry Molarity = mols solute/liters of solution or, in symbolic form: M = n/V or, rearranging the equation: n = M x V Thus, 0.346 mol of HCl dissolved to make 550 mL of solution has a molarity of: MHCl = 0.346 mol/0.550 L = 0.629 mol/L Solution Stoichometry Sometimes, a concentrated solution must be diluted for use in an experiment and the concentration of the diluted solution is needed. The key to this calculation is the fact that if a sample of a concentrated solution is diluted the number of mols of solute does not change, just the volume of the solution changes. This can be represented symbolically: or Mi ni = nf x Vi = Mf x Vf where i is initial and f is final Solution Stoichometry What will be the final volume of a solution if 10 mL of a 1.5 M solution is to be diluted to give a 0.30 M solution? Mi x Vi = Mf x Vf Vf = Mi x Vi / Mf = 1.5 M x 10 mL / 0.30 M = 50 mL Solution Stoichometry How many grams of NaCl are required to precipitate most of the Ag+ ions from 2.50 x 102 mL of 0.0113 M AgNO3 solution? Cl- + Ag+ AgCl mols Ag+ = 0.0113 mol/L x 0.250 L = 0.00283 mol mols Cl- = 0.00283 mol Ag+ x 1 mol Cl- / 1 mol Ag+ = 0.00283 mol mass NaCl = 0.00283 mol x 58.44 g/mol = 0.165 g Solution Stoichometry A 3.664 g sample of a monoprotic acid was dissolved in water. It took 20.27 mL of a 0.1578 M NaOH solution to neutralize the acid. What is the molar mass of the acid? HA + NaOH NaA + H2O mols NaOH = 0.1578 mol/L x 0.02027 L = 0.003199 mol mols HA = 0.003199 mol NaOH x 1mol HA / 1 mol NaOH = 0.003199 mol molar mass = mass/mols = 3.664/0.003199 = 1145 g/mol Reaction Types Precipitation Reactions Some ionic compounds are very soluble such as NaCl while others are only soluble to an extremely small extent .. so much so that we think of them as insoluble. If a solution is prepared with the cations an anions of an insoluble ionic compound in it, the ions will combine to form the solid form of the compound in the solvent and will precipitate out of solution. Reaction Types Precipitation Reactions In order to represent these types of reactions we use a shortened version of the balanced equation called the net ionic equation. Suppose we mix a solution of Pb(NO3)2 and NaI. In each of these solutions there is dissolved a strong electrolyte, however, when mixed there will be Pb+2 ions and I- ions in solution and PbI2 is an insoluble compound .. a precipitate of PbI2 will form: Pb+2 + 2NO3- + 2Na+ + 2IThe net ionic equation is: Pb+2 + 2I- PbI2 2Na+ + 2NO3- + PbI2 Reaction Types Soluble Insoluble Compounds that contain an alkali metal cation or ammonium ion Compounds that contain the nitrate ion Most acetate derived compounds except Most chlorides, bromides, except iodides Most sulfate compounds except silver acetate Ag+, Hg22+, Pb2+ with cations Ba2+, Pb2+, Ag+, Ca2+ Reaction Types Soluble Insoluble Most carbonate and But not phosphate compounds with with alkali metal ions or ammonium ions other metal cations Hydroxides and sulfides But not with alkali metal ions or with ammonium ion and Ca2+, Sr2+, Ba2+ other metal cations Reaction Types Indicate whether or not the following compounds are soluble: 1. Ca3(PO4)2 insoluble 2. Mn(OH)2 insoluble 3. K2S soluble 4. ZnSO4 soluble 5. Hg(NO3)2 soluble Reaction Types Acid-Base Reactions A definition: Acid: a substance that dissociates in water to produce hydrogen ions (and an anion): HA H+ + A- where A- stands for an anion of some type such as Clor NO3-. Base: a substance that dissociates in water to produce hydroxide ions (and a cation): MOH M+ + OH- Reaction Types Acid-Base Reactions The Bronsted-Lowry definition: Acid: a substance that donates a proton to a base: HA + B HB+ + A- Base: a substance that accepts a proton from an acid: HA + B HB+ + A- acid conjugate base base conjugate acid Reaction Types Acid-Base Reactions H2O + H2O H3O+ + OH- H3O+ and H2O are a conjugate acid-base pair as are OH- and H2O. Reaction Types Oxidation-Reduction (Redox) Reactions These reactions are ones in which there is a transfer of electrons from one molecule to another resulting in a change of oxidation number on some of the atoms involved: 2Na + Cl2 2NaCl In this case the oxidation number on Na and Cl2 are zero (remember the rules for assigning oxidation numbers?) and the oxidation number of Na in NaCl is +1 and on Cl in NaCl is -1. The oxidation number of Na has increased and we say that it has been oxidized ... the oxidation number of Cl has decreased and we say it has been reduced. So, if an atom's oxidation number increases or it formally loses electrons it has been oxidized and if an atom's oxidation number decreases or it formally gains electrons it has been reduced. Reaction Types Half Reactions In order to explicitly show the gain and loss of electrons we can split the redox reaction into half reactions: Na 2e- + Na+ Cl2 + e2Cl- (shows oxidation reaction) (shows reduction reaction) In order for the overall reaction to be balanced the two half reactions must add together so that there are no electrons in the equation. This means that for the above example we would have to multiply the first reaction by 2 in order to cancel out the numbers of electrons. Half reactions are useful for balancing redox equations, especially the more complex ones. Reaction Types MnO4-(aq) + Br-(aq) acid solution) ---> Mn+2(aq) + Br2(aq) (in 1. Separate the reactants and products into the appropriate oxidation and reduction half reactions. 2. Balance the numbers of atoms of the oxidized or reduced species and write the number of electrons involved. 3. Balance the number oxygens (if any) by adding H2O to the appropriate side of the equation. 4. Balance the number of hydrogens by adding H+ to the appropriate side of the equation. 5. If necessary, multiply the equations by the appropriate factors so that the number of electrons is the same in each half reaction. 6. Add the half reactions. Reaction Types 1. MnO4- + 5eMn2+ Br2 + 2e2Br2. 8H+ +MnO4- + 5e2BrBr2 + 2e3. 16H+ + 2MnO4- + 10e10Br5Br2 + 10e4. 16H+ + 10Br- + 2MnO4- Mn2+ + 4H2O 2Mn2+ + 8H2O 2Mn2+ + 5Br2 + 8H2O The Combined or Ideal Gas Law The three proportionality laws can be combined into one all-encompassing equation: Boyle's Law: rate rate gasA gasB = M wB M wA Charles' Law: V ∝T or V =k ' T Avogadro's Law: V ∝n or V =k ' ' T Combined: nT V∝ P or: V = RnT P More commonly expressed as: PV = nRT where R is the universal gas constant: R = PV/nT Standard Conditions ● ● ● since the volume of a gas varies with pressure and temperature, chemists have agreed on a set of conditions to report our measurements so that comparison is easy – we call these standard conditions – STP standard pressure = 1 atm standard temperature = 273 K – 0°C Molar Mass and Density mols = mass/molar mass or: n= m M substituting into PV = nRT PV = mRT/M solving for M: M= mRT PV Thus, we can find the molar mass of a substance in the gas phase, knowing the mass, temperature, pressure and volume. This allows us to find the molecular formula of a substance if we first determine the empirical formula from mass percent composition data. Molar Mass and Density Similarly: PV = mRT M mRT PM= V or: PM = dRT (density, d = m/V) M = dRT/P Mixtures of Gases ... Dalton's Law John Dalton studied the effect of gases in a mixture. He observed that the total pressure of a gas mixture was the sum of the partial pressure of each gas. Ptotal = P1 + P2 + P3 + .......Pn The partial pressure is defined as the pressure of a single gas in the mixture as if that gas alone occupied the container. In other words, Dalton maintained that since there was an enormous amount of space between the gas molecules within the mixture that the gas molecules did not have any influence on the motion of other gas molecules, therefore the pressure of a gas sample would be the same whether it was the only gas in the container or if it were among other gases. This assumption that molecules act independently of one another works fine as long as there is a lot of space between gas molecules in the mixture and the temperature is not too low. A useful concept when dealing with gas mixtures is mol fraction. Consider, for a mixture of gases at constant V and T: The fraction of mols of gas A is XA = nA/nT. Since V and T are constant XA is also equal to PA/PT. XA is the mol fraction of gas A in the mixture. Collecting Gases ● ● ● ● gases are often collected by having them displace water from a container the problem is that since water evaporates, there is also water vapor in the collected gas the partial pressure of the water vapor, called the vapor pressure, depends only on the temperature – so you can use a table to find out the partial pressure of the water vapor in the gas you collect if you collect a gas sample with a total pressure of 758.2 mmHg* at 25°C, the partial pressure of the water vapor will be 23.78 mmHg – so the partial pressure of the dry gas will be 734.4 mmHg – Table 5.4* Example Problems In alcohol fermentation, yeast converts glucose to ethanol and carbon dioxide: C6H12O6 2C2H5OH + 2CO2 If 5.97 g of glucose are reacted and 1.44 L of CO2 gas are collected at 293K and 0.984 atm, what is the percent yield? Mols glucose = 5.97 g / 180.156 g/mol = 0.0331 mol mols CO2 = 0.0331 mol glucose x 2 mol CO2 / 1 mol glucose = 0.0663 mol (theoretical yield) mols CO2 (actual yield) = PV/RT = (0.984 atm x 1.44 L)/( 0.0821 x 293 K) = 0.0589 mol % yield = (0.0589 mol / 0.0663 mol) x 100 = 88.8% Example Problems At 741 Torr and 44 oC, 7.10 g of a gas occupy a volue of 5.40 L. What is the molar mass of the gas? M = mRT/PV = 7.10 g x 0.0821 Latm/molK x 317 K / (741/760) atm x 5.40L = 35.1 g/mol Example Problems A mixture of gases contains 0.75 mol N2, 0.30 mol O2 and 0.15 mol CO2. If the total pressure of of the mixture is 1.56 atm what are the partial pressures of each gas? The mole fraction of each gas may be used here: total number of mols = 0.75 + 0.30 + 0.15 = 1.2 mols XN2 = nN2/nT = PN2/PT PN2 = (nN2/nT) x PT = (0.75/1.2) x 1.56 atm = 0.98 atm similarly: PO2 = (0.30/1.2) x 1.56 atm = 0.39 atm PCO2 = (0.15/1.2) x 1.56 atm = 0.20 atm Example Problems A gaseous mixture of O2 and Kr has a density of 1.104 g/L at 435 torr and 300K. What is the mol fraction of O2 in the mixture? D pure O2 = MP/RT = 31.999 g/mol x (435/760) atm / 0.0821 x 300 = 0.744 g/L D pure Kr = 83.8 g/mol x (435/760) atm / 0.0821 x 300 = 1.95 g/L Dmixture = XO2 x DO2 + XKr x DKr = XO2 x DO2 + (1-XO2) x DKr XO2 = (Dmix – DKr)/(DO2 – DKr) = (1.104 – 1.95)/(0.744 – 1.95) = 0.70 Molecular Velocities • all the gas molecules in a sample can travel at different speeds • however, the distribution of speeds follows a pattern called a Boltzman distribution • we talk about the “average velocity” of the molecules, but there are different ways to take this kind of average • the method of choice for our average velocity is called the root-mean-square method, where the rms average velocity, urms, is the square root of the average of the sum of the squares of all the molecule velocities r a tgea s A M w B = r a tgea s B M w A Effusion Graham’s Law of Effusion • for two different gases at the same temperature, the ratio of their rates of effusion is given by the following equation: rate gasA M wB = rate gasB M wA Temperature and Molecular Velocities • KEavg = ½NAmu2 (for one mol of gas particles) NA is Avogadro’s number • KEavg = 1.5RT R is the gas constant in energy units, 8.314 J/mol∙K 1 J = 1 kg∙m2/s2 • equating and solving we get: NA∙mass = molar mass in kg/mol rate rate • gasA gasB = M wB M wA as temperature increases, the average velocity increases Ideal vs. Real Gases • • • Real gases often do not behave like ideal gases at high pressure or low temperature Ideal gas laws assume 1) no attractions between gas molecules 2) gas molecules do not take up space based on the kinetic-molecular theory at low temperatures and high pressures these assumptions are not valid The Effect of Molecular Volume ● ● ● at high pressure, the amount of space occupied by the molecules is a significant amount of the total volume the molecular volume makes the real volume larger than the ideal gas law would predict van der Waals modified the ideal gas equation to account for the molecular volume – b is called a van der Waals constant and is different for every gas because their molecules are different sizes nRT V= n b P or (V −n b )= nRT P The Effect of Intermolecular Attractions ● ● ● at low temperature, the attractions between the molecules is significant the intermolecular attractions makes the real pressure less than the ideal gas law would predict van der Waals modified the ideal gas equation to account for the intermolecular attractions – a is called a van der Waals constant and is different for every gas because their molecules are different sizes nRT n 2 P= −a( ) V V or n 2 nRT Pa( ) = V V Van der Waals’ Equation ● combining the equations to account for molecular volume and intermolecular attractions we get the following equation – used for real gases – a and b are called van der Waal constants and are different for each gas n 2 ( Pa( ) ×(V −nb)=nRT V