synthesis and characterization of tetracarbonyl[6
... heterocyclic compounds. Although some organometallic compounds have been known for a long time, it is only in the last four or five decades that organometallic chemistry has come into its own and has experienced tremendous growth, both at fundamental level where our insight into the nature of chemic ...
... heterocyclic compounds. Although some organometallic compounds have been known for a long time, it is only in the last four or five decades that organometallic chemistry has come into its own and has experienced tremendous growth, both at fundamental level where our insight into the nature of chemic ...
Section 4.9 Oxidation–Reduction Reactions
... • Limiting Reactant – reactant that is completely consumed and limits amount of product • Reactant in excess – reactant present in greater quantity than limiting reactant • Theoretical Yield – amount of product made based on consumption of all the limiting reactant • Actual Yield – amount of product ...
... • Limiting Reactant – reactant that is completely consumed and limits amount of product • Reactant in excess – reactant present in greater quantity than limiting reactant • Theoretical Yield – amount of product made based on consumption of all the limiting reactant • Actual Yield – amount of product ...
Density functional studies of functionalized graphitic
... The volcano plot in Fig. 4 contains the lines representing the Gibbs free energies of each step in the mechanism, and the tops for ORR (at B1.2 eV) and OER (at B1.5 eV) result from the intersection of those lines. We remark that in Fig. 4 we have only marked the active sites with the highest activit ...
... The volcano plot in Fig. 4 contains the lines representing the Gibbs free energies of each step in the mechanism, and the tops for ORR (at B1.2 eV) and OER (at B1.5 eV) result from the intersection of those lines. We remark that in Fig. 4 we have only marked the active sites with the highest activit ...
Synthesis of Cobalt(III), Iron(III), and Chromium(III) Complexes with
... The Co(III)-based complexes 6a, 6b and 9a were synthesized by treating CoCl2 · 6H2 O with two molar equivalents of the corresponding bidentate ligand in MeOH, followed by oxidation with H2 O2 (Scheme 1). The Fe(III)- (7a, 7b, 10a and 10b) and Cr(III)-based complexes (8a, 8b, 11a, and 11b) were synth ...
... The Co(III)-based complexes 6a, 6b and 9a were synthesized by treating CoCl2 · 6H2 O with two molar equivalents of the corresponding bidentate ligand in MeOH, followed by oxidation with H2 O2 (Scheme 1). The Fe(III)- (7a, 7b, 10a and 10b) and Cr(III)-based complexes (8a, 8b, 11a, and 11b) were synth ...
FREE Sample Here
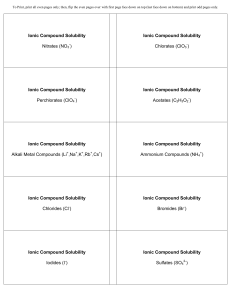
... http://testbankwizard.eu/Test-Bank-for-Biology-with-MasteringBiology-8th-Edition-by-Campbe ll 45) Nitrogen (N) is much more electronegative than hydrogen (H). Which of the following statements is correct about the atoms in ammonia (NH3)? A) Each hydrogen atom has a partial positive charge. B) The ni ...
... http://testbankwizard.eu/Test-Bank-for-Biology-with-MasteringBiology-8th-Edition-by-Campbe ll 45) Nitrogen (N) is much more electronegative than hydrogen (H). Which of the following statements is correct about the atoms in ammonia (NH3)? A) Each hydrogen atom has a partial positive charge. B) The ni ...
communications - University of Notre Dame
... the number of required countercations by substitution with an atom of an electron-poorer element. This approach was used in an attempt to substitute a germanium atom of Ge94ÿ by zinc and led to the synthesis of Cs6Ge8Zn.[6] In this compound two eclipsed germanium tetrahedra are interconnected by the ...
... the number of required countercations by substitution with an atom of an electron-poorer element. This approach was used in an attempt to substitute a germanium atom of Ge94ÿ by zinc and led to the synthesis of Cs6Ge8Zn.[6] In this compound two eclipsed germanium tetrahedra are interconnected by the ...