Preview Sample 2
... Joseph Proust showed that when elements combine to form new substances, they do so in specific mass ratios. According to the law of multiple proportions, when water forms, the mass ratio of hydrogen to oxygen is variable. John Dalton's atomic theory disagreed with the ancient Greek philosophers' ide ...
... Joseph Proust showed that when elements combine to form new substances, they do so in specific mass ratios. According to the law of multiple proportions, when water forms, the mass ratio of hydrogen to oxygen is variable. John Dalton's atomic theory disagreed with the ancient Greek philosophers' ide ...
FREE Sample Here - We can offer most test bank and
... c. law of constant composition b. law of conservation of mass d. all of the above ANS: C PTS: 1 TOP: 2.3 - WHAT ARE THE POSTULATES OF DALTON’S ATOMIC THEORY? 26. Which of the following statements, all of which were part of Dalton’s atomic theory, was later shown to be false? a. All matter is made up ...
... c. law of constant composition b. law of conservation of mass d. all of the above ANS: C PTS: 1 TOP: 2.3 - WHAT ARE THE POSTULATES OF DALTON’S ATOMIC THEORY? 26. Which of the following statements, all of which were part of Dalton’s atomic theory, was later shown to be false? a. All matter is made up ...
Chemistry M.4 Lesson 1 Atom and Periodic Table
... Bohr used the term energy levels (or shells) to describe. He said that the energy of an electron is quantized, meaning electrons can have one energy level or another but nothing in between. The energy level an electron normally occupies is called ground state. But it can move to a higherenergy (l ...
... Bohr used the term energy levels (or shells) to describe. He said that the energy of an electron is quantized, meaning electrons can have one energy level or another but nothing in between. The energy level an electron normally occupies is called ground state. But it can move to a higherenergy (l ...
Chapter 22 - 2012 Book Archive
... uses. Both indium and thallium oxides are released in flue dust when sulfide ores are converted to metal oxides and SO2. Until relatively recently, these and other toxic elements were allowed to disperse in the air, creating large “dead zones” downwind of a smelter. The flue dusts are now trapped an ...
... uses. Both indium and thallium oxides are released in flue dust when sulfide ores are converted to metal oxides and SO2. Until relatively recently, these and other toxic elements were allowed to disperse in the air, creating large “dead zones” downwind of a smelter. The flue dusts are now trapped an ...
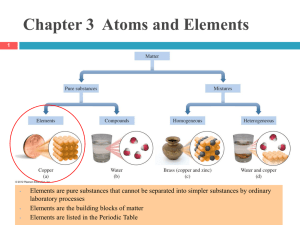
Elements and the Periodic Table
... •The elements below the lanthanides are called actinides. Many of these elements are so unstable that they last for only a fraction of a second after they are made. •The metals that follow uranium are man-made (synthetic) by forcing nuclear particles to collide with one another. ...
... •The elements below the lanthanides are called actinides. Many of these elements are so unstable that they last for only a fraction of a second after they are made. •The metals that follow uranium are man-made (synthetic) by forcing nuclear particles to collide with one another. ...
goyal brothers prakashan
... 6. Rutherford’s atomic model proposed that a very, very small nucleus is present inside the atom and the electrons revolve around it in fixed orbits or shells, much like the planets revolve around the sun. The stability of the atom could not be explained by this model. ...
... 6. Rutherford’s atomic model proposed that a very, very small nucleus is present inside the atom and the electrons revolve around it in fixed orbits or shells, much like the planets revolve around the sun. The stability of the atom could not be explained by this model. ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Chapter 4 Elements and the Periodic Table
... The Group 17 elements, the halogens, are very reactive. Atoms of these elements easily form compounds by sharing or gaining one electron when reacting with atoms of other elements. ...
... The Group 17 elements, the halogens, are very reactive. Atoms of these elements easily form compounds by sharing or gaining one electron when reacting with atoms of other elements. ...
Chapter 4 Elements and the Periodic Table
... The Group 17 elements, the halogens, are very reactive. Atoms of these elements easily form compounds by sharing or gaining one electron when reacting with atoms of other elements. ...
... The Group 17 elements, the halogens, are very reactive. Atoms of these elements easily form compounds by sharing or gaining one electron when reacting with atoms of other elements. ...
Chemistry
... Materials and Technology Chemical research and development in the twentieth century have provided us with new materials that have profoundly improved the quality of our lives and helped to advance technology in countless ways. A few examples are polymers (including rubber and nylon), ceramics (such ...
... Materials and Technology Chemical research and development in the twentieth century have provided us with new materials that have profoundly improved the quality of our lives and helped to advance technology in countless ways. A few examples are polymers (including rubber and nylon), ceramics (such ...
ChemistryReview
... All the atoms of an element have the same atomic number because the atomic number equals the number of protons in an atom. If one of the atoms had a different number of protons, the atom would not be a calcium atom. The mass number can vary because it is the sum of the protons and neutrons, and isot ...
... All the atoms of an element have the same atomic number because the atomic number equals the number of protons in an atom. If one of the atoms had a different number of protons, the atom would not be a calcium atom. The mass number can vary because it is the sum of the protons and neutrons, and isot ...
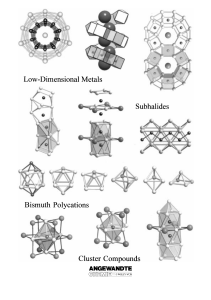
From the Metal to the Molecule
... Michael Ruck was born in Pforzheim (Germany) in 1963 and began to study chemistry at the Universität Karlsruhe in 1984. After finishing his degree in the group of Hartmut Bärnighausen he moved in 1989 to the Max-Planck Institut für Festkörperforschung in Stuttgart. There he joined the group of Arndt ...
... Michael Ruck was born in Pforzheim (Germany) in 1963 and began to study chemistry at the Universität Karlsruhe in 1984. After finishing his degree in the group of Hartmut Bärnighausen he moved in 1989 to the Max-Planck Institut für Festkörperforschung in Stuttgart. There he joined the group of Arndt ...
Surviving Chemistry - Bremen High School District 228
... . Electrons are found outside the nucleus in a region called orbital Orbital is the most probable location of finding an electron with certain energy in an atom. Below is a list of some historical scientists and their proposed models of atom in order from the earliest model to the current model. Des ...
... . Electrons are found outside the nucleus in a region called orbital Orbital is the most probable location of finding an electron with certain energy in an atom. Below is a list of some historical scientists and their proposed models of atom in order from the earliest model to the current model. Des ...
A Chemical Progression
... By extension, he predicted that all matter should be made up of indivisible units, or as he called them, “atomos” (meaning “uncuttable” in Greek). Thus, the existence of what we today call atoms was theoretically predicted thousands of years before there was any experimental evidence regarding them. ...
... By extension, he predicted that all matter should be made up of indivisible units, or as he called them, “atomos” (meaning “uncuttable” in Greek). Thus, the existence of what we today call atoms was theoretically predicted thousands of years before there was any experimental evidence regarding them. ...
No Slide Title - MDC Faculty Home Pages
... Atomic Symbols are used to represent Isotopes Example: Isotopes of Magnesium ...
... Atomic Symbols are used to represent Isotopes Example: Isotopes of Magnesium ...
Holt Modern Chemistry Workbook: intro - ch 5
... CONNECT Basic research can lead to chance discoveries. For example, Ray Plunkett discovered the properties of Teflon® by accident. After removing the gas from a container, the container was still heavier than expected. Inside, he found a white solid with nonstick properties. Sometimes the uses o ...
... CONNECT Basic research can lead to chance discoveries. For example, Ray Plunkett discovered the properties of Teflon® by accident. After removing the gas from a container, the container was still heavier than expected. Inside, he found a white solid with nonstick properties. Sometimes the uses o ...
Has the Periodic Table Been Successfully Axiomatized?
... electrons were embedded in the main body of the atoms and were held to circulate in concentric rings (Thomson, 1904). The particular arrangement of how many electrons should occur in each ring was adapted from the earlier work of an American physicist Mayer, who had experimented by floating small ba ...
... electrons were embedded in the main body of the atoms and were held to circulate in concentric rings (Thomson, 1904). The particular arrangement of how many electrons should occur in each ring was adapted from the earlier work of an American physicist Mayer, who had experimented by floating small ba ...
Nucleon number
... At the end of this topic, students should be able : (a) Identify and describe proton, electron and neutron as subatomic particle. ...
... At the end of this topic, students should be able : (a) Identify and describe proton, electron and neutron as subatomic particle. ...
Document
... all atoms present before the reaction are present after atoms are not created or destroyed, just rearranged therefore the total mass will remain the same – Law of Conservation of Mass ...
... all atoms present before the reaction are present after atoms are not created or destroyed, just rearranged therefore the total mass will remain the same – Law of Conservation of Mass ...
Unit 1 Section 4 - Atomic Structure PPT
... pieces - protons, neutrons and electrons • The nucleus contains protons and neutrons • The nucleus is only about 10-13 cm in diameter • The electrons move outside the nucleus with an average distance of about 10-8 cm ...
... pieces - protons, neutrons and electrons • The nucleus contains protons and neutrons • The nucleus is only about 10-13 cm in diameter • The electrons move outside the nucleus with an average distance of about 10-8 cm ...
The d- and f- Block Element Block Elements The d- and f
... The electronic configurations of Zn, Cd and Hg are represented by the general formula (n-1)d10ns 2. The orbitals in these elements are completely filled in the ground state as well as in their common oxidation states. Therefore, they are not regarded as transition elements. The d orbitals of the tra ...
... The electronic configurations of Zn, Cd and Hg are represented by the general formula (n-1)d10ns 2. The orbitals in these elements are completely filled in the ground state as well as in their common oxidation states. Therefore, they are not regarded as transition elements. The d orbitals of the tra ...
1.9 M - Thierry Karsenti
... 2. Atom: the smallest particle of an element that retains the identify and properties of the element and can take part in a chemical change. 3. Atomic number (symbol Z): the number of protons in the nucleus of each atom. 4. Compound: a substance that is formed when two or more elements combine chemi ...
... 2. Atom: the smallest particle of an element that retains the identify and properties of the element and can take part in a chemical change. 3. Atomic number (symbol Z): the number of protons in the nucleus of each atom. 4. Compound: a substance that is formed when two or more elements combine chemi ...
Chapter 4 Elements and the Periodic Table The Periodic Table
... Metals in the Periodic Table Group 2 of the periodic table contains the alkaline earth metals. These elements are not as reactive as the metals in Group 1, but they are more reactive than most other metals. ...
... Metals in the Periodic Table Group 2 of the periodic table contains the alkaline earth metals. These elements are not as reactive as the metals in Group 1, but they are more reactive than most other metals. ...