BỘ GIÁO DỤC VÀ ĐÀO TẠO - THPT Chuyên Võ Nguyên Giáp
... proton, has a mass of approximately 1 atomic mass unit (amu). When we add the total number of protons and neutrons in the nucleus of an atom, we get the atom’s mass number. Because the neutron has no charge, it does not affect the atomic number and does not alter the identity of the element. For thi ...
... proton, has a mass of approximately 1 atomic mass unit (amu). When we add the total number of protons and neutrons in the nucleus of an atom, we get the atom’s mass number. Because the neutron has no charge, it does not affect the atomic number and does not alter the identity of the element. For thi ...
TOPIC 24 Nucleus - jmr physics website
... 25 A chlorine atom, proton number 17, can gain an electron and become a chlorine ion. A sodium atom, proton number 11, can lose an electron and become a sodium ion. (a) What is the sign of the charge on (i) a chlorine ion, ...
... 25 A chlorine atom, proton number 17, can gain an electron and become a chlorine ion. A sodium atom, proton number 11, can lose an electron and become a sodium ion. (a) What is the sign of the charge on (i) a chlorine ion, ...
Chapter 5 Section 2 Electron Configuration and the Periodic Table
... radium • Group 2 metals are less reactive than the alkali metals, but are still too reactive to be found in nature in pure form. ...
... radium • Group 2 metals are less reactive than the alkali metals, but are still too reactive to be found in nature in pure form. ...
Period:______ Table Number
... 47. Nearly 2000 years ago the Greek philosopher DEMOCRITUS gave us the word atom when he said that all matter was composed of tiny indivisible particles called “atomos.” P. 73, VCR: Atoms and Molecules 48. At the present time about 118 different elements have been discovered and officially recognize ...
... 47. Nearly 2000 years ago the Greek philosopher DEMOCRITUS gave us the word atom when he said that all matter was composed of tiny indivisible particles called “atomos.” P. 73, VCR: Atoms and Molecules 48. At the present time about 118 different elements have been discovered and officially recognize ...
Topic 2.3 The Atom Electron Configuration
... down as much information about each one as you can remember. What can you say about the numbers of protons and electrons in an atom? ...
... down as much information about each one as you can remember. What can you say about the numbers of protons and electrons in an atom? ...
atomic mass
... -Based on natural abundance of isotopes 1)Change % to decimal .1991 and .8009 2)Multiply decimal by the mass 3)Add the numbers together Element X has two isotopes. The isotope with a mass of 10.012 amu has a relative abundance of 19.91%. The isotope with a mass of 11.009 amu has a relative abundance ...
... -Based on natural abundance of isotopes 1)Change % to decimal .1991 and .8009 2)Multiply decimal by the mass 3)Add the numbers together Element X has two isotopes. The isotope with a mass of 10.012 amu has a relative abundance of 19.91%. The isotope with a mass of 11.009 amu has a relative abundance ...
Intro to the Periodic Table
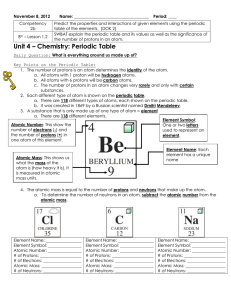
... 7. Find the element with the atomic number of 8. a. What is the element name? _________________________ b. What is the element symbol? _________________________ c. How many protons are in one atom of this element? __________________ d. How many electrons are in one atom of this element? ____________ ...
... 7. Find the element with the atomic number of 8. a. What is the element name? _________________________ b. What is the element symbol? _________________________ c. How many protons are in one atom of this element? __________________ d. How many electrons are in one atom of this element? ____________ ...
Material presented
... • Obtain the total number of electrons in the atom from the atomic number • Every electron has a place to stay • Electrons in atoms occupy the lowest energy orbitals that are available – 1s first • Each principal energy level, n contains only n sublevels • Each sublevel is composed of orbitals • No ...
... • Obtain the total number of electrons in the atom from the atomic number • Every electron has a place to stay • Electrons in atoms occupy the lowest energy orbitals that are available – 1s first • Each principal energy level, n contains only n sublevels • Each sublevel is composed of orbitals • No ...
02 Atomic Structure [ppt 1MB]
... I can describe the basic structure of an atom and state the location and charge of the proton, electron and neutron within the atom structure I can state the relative masses of the proton, neutron and electron. I can explain what is meant by atomic number and state that all elements are arranged in ...
... I can describe the basic structure of an atom and state the location and charge of the proton, electron and neutron within the atom structure I can state the relative masses of the proton, neutron and electron. I can explain what is meant by atomic number and state that all elements are arranged in ...
LEARNING WORKSHEET ON ATOMIC STRUCTURE
... *Acids and bases *Chemical changes in matter (e.g., rusting [slow oxidation], combustion [fast oxidation], food spoilage) ...
... *Acids and bases *Chemical changes in matter (e.g., rusting [slow oxidation], combustion [fast oxidation], food spoilage) ...
Chemistry Semester 1 Exam Review Study Island
... 45. When an electron moves up to a higher orbit, a quantum of light energy is absorbed. This quantum of light energy is also known as a(n) _______. A. alpha particle B. neutron C. quark D. photon ...
... 45. When an electron moves up to a higher orbit, a quantum of light energy is absorbed. This quantum of light energy is also known as a(n) _______. A. alpha particle B. neutron C. quark D. photon ...
ppt
... Mendeleyev’s periodic table (1869) classified and sorted elements based on common chemical properties. His table had 62 known elements, and left space for 20 elements that were not yet discovered. The elements were arranged in order of atomic number. ...
... Mendeleyev’s periodic table (1869) classified and sorted elements based on common chemical properties. His table had 62 known elements, and left space for 20 elements that were not yet discovered. The elements were arranged in order of atomic number. ...
Oxidation Numbers and Ionic Compounds
... 1. Count the total number of valence e-. 2. Determine the central atom. The following are guides: Often the unique atom (only one of it) is the central atom. Or put the least electronegative element in the middle. 3. Arrange the other atoms around the central atom creating a skeleton. 4. Connect ...
... 1. Count the total number of valence e-. 2. Determine the central atom. The following are guides: Often the unique atom (only one of it) is the central atom. Or put the least electronegative element in the middle. 3. Arrange the other atoms around the central atom creating a skeleton. 4. Connect ...
Periodic table Periodic Trends
... An oxide is formed from the combination of an element with oxygen Use the charge of a metal cation to deduce the chemical formula of a metal oxide: • Na+ combines with O2- to form Na2O • Ca2+ combines with O2- to form CaO • Al3+ combines with O2- to form Al2O3 Oxides of metals are basic: they react ...
... An oxide is formed from the combination of an element with oxygen Use the charge of a metal cation to deduce the chemical formula of a metal oxide: • Na+ combines with O2- to form Na2O • Ca2+ combines with O2- to form CaO • Al3+ combines with O2- to form Al2O3 Oxides of metals are basic: they react ...
Chapter 3 Atoms and Elements
... In Dalton's atomic theory, atoms: • are tiny particles of matter • of an element are similar to each other and different from other elements • of two or more different elements combine to form compounds • are rearranged to form new combinations in a chemical reaction ...
... In Dalton's atomic theory, atoms: • are tiny particles of matter • of an element are similar to each other and different from other elements • of two or more different elements combine to form compounds • are rearranged to form new combinations in a chemical reaction ...
Unit #3: ATOMIC STRUCTURE - Miss Virga`s Chemistry Class
... (1) They are positive subatomic particles and are found in the nucleus. (2) They are positive subatomic particles and are found surrounding the nucleus. (3) They are negative subatomic particles and are found in the nucleus. (4) They are negative subatomic particles and are found surrounding the nuc ...
... (1) They are positive subatomic particles and are found in the nucleus. (2) They are positive subatomic particles and are found surrounding the nucleus. (3) They are negative subatomic particles and are found in the nucleus. (4) They are negative subatomic particles and are found surrounding the nuc ...
Build An Atom - ChemConnections
... Neutral atoms have ________________________________ protons and electrons. Positive ions have ________________________________ protons than electrons. Negative ions have _______________________________ protons than electrons. ...
... Neutral atoms have ________________________________ protons and electrons. Positive ions have ________________________________ protons than electrons. Negative ions have _______________________________ protons than electrons. ...
Student Copy Study Guide Introduction to Periodic
... 19.The atomic number of an element is the total number of which particles in the nucleus? a. neutrons b. protons c. electrons d. protons and electrons 20. Who was the man who lived from 460 B.C.–370 B.C. and was among the first to suggest the idea of atoms? a. Atomos b. Dalton c. Democritus d. Thoms ...
... 19.The atomic number of an element is the total number of which particles in the nucleus? a. neutrons b. protons c. electrons d. protons and electrons 20. Who was the man who lived from 460 B.C.–370 B.C. and was among the first to suggest the idea of atoms? a. Atomos b. Dalton c. Democritus d. Thoms ...
Elements, Compounds, and Mixtures
... CHEMICAL COMPOUNDS are composed of atoms and so can be decomposed to those atoms. The red compound is composed of • nickel (Ni) (silver) • carbon (C) (black) • hydrogen (H) (white) • oxygen (O) (red) • nitrogen (N) (blue) ...
... CHEMICAL COMPOUNDS are composed of atoms and so can be decomposed to those atoms. The red compound is composed of • nickel (Ni) (silver) • carbon (C) (black) • hydrogen (H) (white) • oxygen (O) (red) • nitrogen (N) (blue) ...
What do you already know about atoms?
... • Since the electrons on the inner shells are buried they cannot leave the atom. • Which would be the only electrons that can detach from the atom? • The one in the last layer! • They are also the most loosely attached to the nucleus because they are so far away. ...
... • Since the electrons on the inner shells are buried they cannot leave the atom. • Which would be the only electrons that can detach from the atom? • The one in the last layer! • They are also the most loosely attached to the nucleus because they are so far away. ...
1. Atomic Structure
... Atoms are very small – they are about 0.00000001 cm wide. Think about the thickness of a crisp. The number of atoms you would need to stack up to make the thickness of a crisp, is approximately the same number of crisps you would need to stack up to make the height of Mount Everest! ...
... Atoms are very small – they are about 0.00000001 cm wide. Think about the thickness of a crisp. The number of atoms you would need to stack up to make the thickness of a crisp, is approximately the same number of crisps you would need to stack up to make the height of Mount Everest! ...
Chapter 4 Atoms and Elements
... • The properties of atoms determine the properties of matter. • An atom is the smallest identifiable unit of an element. • An element is a substance that cannot be broken down into simpler substances. • There are about 91 different elements in nature, and consequently about 91 different kinds of ato ...
... • The properties of atoms determine the properties of matter. • An atom is the smallest identifiable unit of an element. • An element is a substance that cannot be broken down into simpler substances. • There are about 91 different elements in nature, and consequently about 91 different kinds of ato ...
Powerpoint covering atomic structure and isotopes
... Atoms are very small – they are about 0.00000001 cm wide. Think about the thickness of a crisp. The number of atoms you would need to stack up to make the thickness of a crisp, is approximately the same number of crisps you would need to stack up to make the height of Mount Everest! ...
... Atoms are very small – they are about 0.00000001 cm wide. Think about the thickness of a crisp. The number of atoms you would need to stack up to make the thickness of a crisp, is approximately the same number of crisps you would need to stack up to make the height of Mount Everest! ...
Ch 04 AtomicStructure
... Evidence about Dalton’s atomic theory has shown that A. all of Dalton's hypotheses were correct. B. atoms of an element can have different numbers of protons. C. atoms are divisible. D. all atoms of an element are not identical but they must all have the same mass. Why did J. J. Thomson reason that ...
... Evidence about Dalton’s atomic theory has shown that A. all of Dalton's hypotheses were correct. B. atoms of an element can have different numbers of protons. C. atoms are divisible. D. all atoms of an element are not identical but they must all have the same mass. Why did J. J. Thomson reason that ...
Midterm Review Teacher Answer Key December 21, 2011 `see
... Use the Periodic Table of the Elements. Radon (Rn, atomic number 86) is a noble gas. It is found in Group 18. Elements in Group 18 have completed outer (valence) shells of electrons and do not readily form compounds. [1 point] 'see explanation below' 31. Base your answer on the information below. Th ...
... Use the Periodic Table of the Elements. Radon (Rn, atomic number 86) is a noble gas. It is found in Group 18. Elements in Group 18 have completed outer (valence) shells of electrons and do not readily form compounds. [1 point] 'see explanation below' 31. Base your answer on the information below. Th ...





![02 Atomic Structure [ppt 1MB]](http://s1.studyres.com/store/data/000821172_1-5bf1afd152b32026d524139a10b8292f-300x300.png)