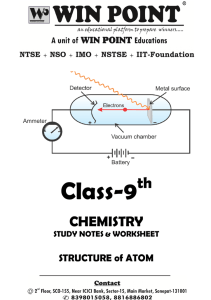
Chapter 17 Resource: Properties of Atoms and the Periodic Table
... 1. List 20 of your favorite foods and drinks. 2. Describe basic characteristics of each of your food and drink items. For example, you might describe the primary ingredient, nutritional value, taste, and color of each item. You also could identify the food group of each item such as fruits/vegetable ...
... 1. List 20 of your favorite foods and drinks. 2. Describe basic characteristics of each of your food and drink items. For example, you might describe the primary ingredient, nutritional value, taste, and color of each item. You also could identify the food group of each item such as fruits/vegetable ...
Isi dengan Judul Halaman Terkait Adaptif
... Charged proton = + 1,6 x 10-19 C Neutron is uncharged particle which has mass ± 1,67 x 10-27 kg ...
... Charged proton = + 1,6 x 10-19 C Neutron is uncharged particle which has mass ± 1,67 x 10-27 kg ...
The Atom
... nucleus electrons electron clouds OBJ ECTIVES ! Describe some of the experiments that led to the current atomic theory. ! Compare the different models of the atom. ! Explain how the atomic theory has changed as scientists have discovered new information about the atom. ...
... nucleus electrons electron clouds OBJ ECTIVES ! Describe some of the experiments that led to the current atomic theory. ! Compare the different models of the atom. ! Explain how the atomic theory has changed as scientists have discovered new information about the atom. ...
File
... The results of these experiments are summarised below: (a) The cathode rays start from cathode and move towards the anode. (b) These rays themselves are not visible but their behaviour can be observed with the help of certain kind of materials (fluorescent or phosphorescent) which glow when hit by t ...
... The results of these experiments are summarised below: (a) The cathode rays start from cathode and move towards the anode. (b) These rays themselves are not visible but their behaviour can be observed with the help of certain kind of materials (fluorescent or phosphorescent) which glow when hit by t ...
Preview Sample 1
... B) protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms. C) outer-shell electrons of two atoms are shared so as to satisfactorily fill the outer electron shells of both atoms. D) outer-shell electrons of one atom are transferred to the inner electron shells of ...
... B) protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms. C) outer-shell electrons of two atoms are shared so as to satisfactorily fill the outer electron shells of both atoms. D) outer-shell electrons of one atom are transferred to the inner electron shells of ...
Elements and the Periodic Table
... The Metalloids • The metalloids have some characteristics of both metals and nonmetals. The most useful property of the metalloids is their varying ability to conduct electricity. • Semiconductors are substances that can conduct electricity. • They are used to make computer chips, transistors, and ...
... The Metalloids • The metalloids have some characteristics of both metals and nonmetals. The most useful property of the metalloids is their varying ability to conduct electricity. • Semiconductors are substances that can conduct electricity. • They are used to make computer chips, transistors, and ...
Elements and the Periodic Table
... The Bohr-Rutherford model and the electron cloud model look very different. To develop his model, Bohr used the concept of forces of attraction between positive and negative charges. This method allowed him to calculate the radius of the electron shells around the nucleus of a hydrogen atom. Schrödi ...
... The Bohr-Rutherford model and the electron cloud model look very different. To develop his model, Bohr used the concept of forces of attraction between positive and negative charges. This method allowed him to calculate the radius of the electron shells around the nucleus of a hydrogen atom. Schrödi ...
Introductory Chemistry, 2nd Edition Nivaldo Tro - Tutor
... very unreactive, practically inert very hard to remove electron from or give an electron to Tro's Introductory Chemistry, Chapter 4 ...
... very unreactive, practically inert very hard to remove electron from or give an electron to Tro's Introductory Chemistry, Chapter 4 ...
85 Q.1 A substance X melts at 1600oC. Its does
... their atoms have the same number of electron shells. their atoms have the same number of electrons in their outermost shells. their atoms have the same electronic arrangement. ...
... their atoms have the same number of electron shells. their atoms have the same number of electrons in their outermost shells. their atoms have the same electronic arrangement. ...
Phosphorus - Jimmy Lai
... Atomic Name For P is Phosphorus Atomic Number For Phosphorus is 15 Atomic Mass For Phosphorus is 30.97376 ...
... Atomic Name For P is Phosphorus Atomic Number For Phosphorus is 15 Atomic Mass For Phosphorus is 30.97376 ...
Elements Compounds
... Ionic bond – electron from Na is transferred to Cl, this causes a charge imbalance in each atom. The Na becomes (Na+) and the Cl becomes (Cl-), charged particles or ions. ...
... Ionic bond – electron from Na is transferred to Cl, this causes a charge imbalance in each atom. The Na becomes (Na+) and the Cl becomes (Cl-), charged particles or ions. ...
Chapter 6: The Periodic Table and Periodic Law
... who is shown in Figure 6-2, proposed an organization scheme for the elements. Newlands noticed that when the elements were arranged by increasing atomic mass, their properties repeated every eighth element. In other words, the first and eighth elements had similar properties, the second and ninth el ...
... who is shown in Figure 6-2, proposed an organization scheme for the elements. Newlands noticed that when the elements were arranged by increasing atomic mass, their properties repeated every eighth element. In other words, the first and eighth elements had similar properties, the second and ninth el ...
elements of chemistry unit
... For Al: Aluminum is a pure element so it has a + 0 oxidation number. For O2: Oxygen is a pure element so it has a + 0 oxidation number. For Al2O3: Oxygen is a group 16 element, so each oxygen atom has a – 2 oxidation number. Since there are 3 oxygen atoms in Al2O3, the O3 atoms have a combined – 6 o ...
... For Al: Aluminum is a pure element so it has a + 0 oxidation number. For O2: Oxygen is a pure element so it has a + 0 oxidation number. For Al2O3: Oxygen is a group 16 element, so each oxygen atom has a – 2 oxidation number. Since there are 3 oxygen atoms in Al2O3, the O3 atoms have a combined – 6 o ...
Chapter 7 Periodic Properties of the Elements
... Development of Periodic Table Dmitri Mendeleev and Lothar Meyer independently came to the same conclusion about how elements should be grouped. ...
... Development of Periodic Table Dmitri Mendeleev and Lothar Meyer independently came to the same conclusion about how elements should be grouped. ...
Introductory Chemistry, 2nd Edition Nivaldo Tro
... • In a chemical change, the number of protons in the nucleus of the atom doesn’t change. (Nuclear reactions can change the number of protons. We will not be concerned with nuclear reactions.) • Atoms can gain or lose electrons to become electrically charged, these particles are called ...
... • In a chemical change, the number of protons in the nucleus of the atom doesn’t change. (Nuclear reactions can change the number of protons. We will not be concerned with nuclear reactions.) • Atoms can gain or lose electrons to become electrically charged, these particles are called ...
File
... The nucleus is very small, but it is densely packed with matter. The SI unit for the mass of an atom is the atomic mass unit (amu). One atomic mass unit equals the mass of a proton, which is about 1.7 ⇥ 10 24 g. Each neutron also has a mass of 1 amu. Therefore, the sum of the protons and neutrons in ...
... The nucleus is very small, but it is densely packed with matter. The SI unit for the mass of an atom is the atomic mass unit (amu). One atomic mass unit equals the mass of a proton, which is about 1.7 ⇥ 10 24 g. Each neutron also has a mass of 1 amu. Therefore, the sum of the protons and neutrons in ...
Chapter 4- Elements and the Periodic Table
... mass of just one proton. A proton and a neutron are about equal in mass. Together, the protons and neutrons make up nearly all the mass of an atom. Figure 8 compares the charges and masses of the three atomic particles. Atoms are too small to be described easily by everyday units of mass, such as gr ...
... mass of just one proton. A proton and a neutron are about equal in mass. Together, the protons and neutrons make up nearly all the mass of an atom. Figure 8 compares the charges and masses of the three atomic particles. Atoms are too small to be described easily by everyday units of mass, such as gr ...
Acquiring the Foundation: The Periodic Table for Middle
... neutron, it has no electrical It simply adds mass to an atom and weighs very nearly the same as a proton” (Gallant 23). The Chadwick, Rutherford and Thomson discoveries provided a physical yet schematic view of the atom and its characteristics: An atom normally has the same number of electrons as pr ...
... neutron, it has no electrical It simply adds mass to an atom and weighs very nearly the same as a proton” (Gallant 23). The Chadwick, Rutherford and Thomson discoveries provided a physical yet schematic view of the atom and its characteristics: An atom normally has the same number of electrons as pr ...
9th class bridge course 74-112
... atom used a beam of 1) -particles which impinged on a metal foil and got absorbed. 2) -rays which impinged on a metal foil and ejected electrons. 3) Helium atoms, which impinged on a metal foil and got scattered. 4) Helium nuclei which impinged on a metal foil and got scattered. 8. Assertion A : ...
... atom used a beam of 1) -particles which impinged on a metal foil and got absorbed. 2) -rays which impinged on a metal foil and ejected electrons. 3) Helium atoms, which impinged on a metal foil and got scattered. 4) Helium nuclei which impinged on a metal foil and got scattered. 8. Assertion A : ...
0 Review Presentations
... ▸ “Atoms comes from Greek word “Atomos,”meaning “indivisible.” ▸ In 1897, J.J. Thompson created the Cathrode Ray Tube that discovered the ...
... ▸ “Atoms comes from Greek word “Atomos,”meaning “indivisible.” ▸ In 1897, J.J. Thompson created the Cathrode Ray Tube that discovered the ...
EL Study Notes
... In 1911, Rutherford proposed that the atom has at its centre a very small positively charged nucleus, which contains almost all the mass of the atom. This nucleus is tiny and the rest of the atom is mostly empty space. (If you magnified an atom to the size of a football stadium, its nucleus would be ...
... In 1911, Rutherford proposed that the atom has at its centre a very small positively charged nucleus, which contains almost all the mass of the atom. This nucleus is tiny and the rest of the atom is mostly empty space. (If you magnified an atom to the size of a football stadium, its nucleus would be ...
Elements and the Periodic Table
... The Metalloids • The metalloids have some characteristics of both metals and nonmetals. The most useful property of the metalloids is their varying ability to conduct electricity. • Semiconductors are substances that can conduct electricity. • They are used to make computer chips, transistors, and ...
... The Metalloids • The metalloids have some characteristics of both metals and nonmetals. The most useful property of the metalloids is their varying ability to conduct electricity. • Semiconductors are substances that can conduct electricity. • They are used to make computer chips, transistors, and ...
Unit 2: Atomic Concepts and Periodic Table (Level 1)
... beginning to learn is the significance of elements within the same column (vertical) and row (horizontal) on the table. Every element found within a given row, or period, has the same number of electron shells, or principle energy levels. Despite this one common feature, atoms of one element within ...
... beginning to learn is the significance of elements within the same column (vertical) and row (horizontal) on the table. Every element found within a given row, or period, has the same number of electron shells, or principle energy levels. Despite this one common feature, atoms of one element within ...
Atoms and Molecules
... the elements hadn’t been discovered yet. Although Mendeleev is credited with developing the Periodic Table, many scientists contributed to its development. The organization was done in such a way that as new elements have been discovered, they fit right where they are supposed to on the Periodic Tab ...
... the elements hadn’t been discovered yet. Although Mendeleev is credited with developing the Periodic Table, many scientists contributed to its development. The organization was done in such a way that as new elements have been discovered, they fit right where they are supposed to on the Periodic Tab ...
Atoms and Molecules
... the elements hadn’t been discovered yet. Although Mendeleev is credited with developing the Periodic Table, many scientists contributed to its development. The organization was done in such a way that as new elements have been discovered, they fit right where they are supposed to on the Periodic Tab ...
... the elements hadn’t been discovered yet. Although Mendeleev is credited with developing the Periodic Table, many scientists contributed to its development. The organization was done in such a way that as new elements have been discovered, they fit right where they are supposed to on the Periodic Tab ...