* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Sorting the Fatty Acid Chaff from the Toxin Wheat, or is it All
Silencer (genetics) wikipedia , lookup
Gene expression wikipedia , lookup
Western blot wikipedia , lookup
Protein moonlighting wikipedia , lookup
Histone acetylation and deacetylation wikipedia , lookup
P-type ATPase wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Expanded genetic code wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Magnesium transporter wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Genetic code wikipedia , lookup
Protein adsorption wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Peptide synthesis wikipedia , lookup
Bottromycin wikipedia , lookup
Molecular evolution wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Point mutation wikipedia , lookup
Biochemistry wikipedia , lookup
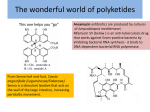
Proceedings 15th ICHA, accepted manuscript Sorting the Fatty Acid Chaff from the Toxin Wheat, or is it All Wheat? Assigning Dinoflagellate PKS genes to Toxin Synthesis Allen Place Institute of Marine and Environmental Technology, University of Maryland Center for Environmental Sciences, 701 E. Pratt Street, Baltimore, MD, USA 21202, [email protected] Abstract Success in identifying genes and enzymes that are involved in the biosynthesis of toxins by dinoflagellates has been limited thus far, despite considerable efforts by many groups. The chemical structures of dinoflagellate polyketides suggest that they are produced by modular type I PKS enzymes in some cases with an involvement of a NRPS, for instance in the case of DTX-5a/5b and spirolides. Unfortunately, dinoflagellates also make fatty acids using PKS machinery so it is difficult to discern the machinery involved in toxin synthesis from those involved in fatty acid synthesis. However, we believe there are several research avenues that can be pursued to open the door to this unique biosynthetic machinery. Given the light dependency of karlotoxin production we hypothesize the starter unit for karlotoxin and amphidinol biosynthesis is glycolate that comes from photorespiration. We argue that the acyl carrier protein and acyl transferase (loader) will be different from that used in fatty acid synthesis because the starter unit is different (i.e. glycolate vs acetate). Focusing only on the acyl carrier proteins and acyl transferases we have found two candidate proteins that we believe are involved in the initiation of karlotoxin synthesis. Keywords: karlotoxin, polyketides, dinoflagellate, biosynthesis Introduction The chemical structures of polyketides from dinoflagellates suggest that they are produced by type I polyketide synthases (PKSs), and in some cases with the involvement of a non-ribosomal peptide synthetase (NRPSs). A minimal PKS has an acyltransferase (AT) domain, a β-ketosynthase(KS) domain, and an acyl carrier protein (ACP). The AT domain covalently transfers a specific carboxylic acid from acyl-CoA to the ACP, which is then condensed by the KS domain to another ACP-bound acyl chain. A PKS module may have optional β-ketoacyl reductase (KR), dehydrogenase (DH), and enoyl reductase (ER) domains, which reduce the β-ketone to an alcohol, dehydrate the alcohol, and saturate the resultant double bond, respectively. In analogy, a minimal NRPS provides an adenylation domain (A), which specifically activates an amino acid, a peptidyl carrier protein (PCP), and a condensation domain (C) that creates a peptide bond between two PCP-bound amino acids. Thioesterase (TE) domains may release, and cyclize the final enzyme products. Type I PKSs and NRPSs usually consist of large, non-iterative, multidomain enzymes. Modular type I PKSs and NRPSs form megasynthetases that generally follow a colinearity rule, where one module extends a growing acyl or peptidyl chain by one particular unit. Each PKS module carries a set of catalytic domains that perform one round of polyketide elongation and modification. During these processes, an AT domain attaches the corresponding acyl-CoA building block onto an acyl carrier protein domain (ACP), a KS domain elongates the polyketide chain with this acyl unit and optional additional domains further modify the resulting intermediate. An example of this modular synthesis is presented in Fig. 1 for the first 14 carbons of karlotoxin with glycolate being the starter unit rather than acetate. For these 14 carbons, 8 KS and AT domains are required with only 7 KRs and 3 and 2 DHs and ERs modules. However, these 8 modular genes have not been found in any karlotoxin producing species. The frequently observed close correlation between the domain architecture and the sequence of functional groups in the polyketide chain, codified as the ‘colinearity rule’, has enabled direct prediction of polyketide structures from genomic sequences and vice versa. However, this colinearity appears to be broken in dinoflagellates. Kubota et al. (2006) screened genomic DNA from five amphidinolide-producing and eight nonproducing dinoflagellate strains by degenerate PCR for the presence of β-ketosynthase (KS) domains of type I PKS genes. Fragments of Proceedings 15th ICHA, accepted manuscript Fig. 1. Predicted PKS modular structure for the first 14 carbons of karlotoxin. The starter unit was glycolate rather than acetate. fourteen unique KS domains were detected. These sequences were exclusively present in amphidinolide producer strains, and a genomic fosmid DNA library was constructed from the amphidinolide-producing strain Amphidinium sp. Y-42. Kubota et al. (2006) detected a single clone out of a total of 100,000 PCR-screened clones, which harbored PKS-related sequences, and the entire fosmid insert (36.4 kb) was sequenced. The fosmid insert had six sequence regions, KS, AT, DH, KR, ACP, and TE that were related to type I PKS genes. Their genomic arrangement was unusual however, as several frame-shifts occurred within and between catalytic domains. The proteincoding region was flanked on both sides by long stretches of non-coding sequence, and the mid section of the protein-coding region contained a 4 kb stretch of sequence that presumably represented an intron. Only approximately 15% of the 36.4 kb long fosmid insert consisted of protein-coding sequence. This sequence encoded putative catalytic functions for only a single elongation cycle of a 26-membered polyketide. If one would extrapolate based on these data, all genes required for the production of amphidinolide may occupy up to 500 kb of genomic DNA. Further, it would not be certain, whether they were present on the same locus, or distributed throughout the genome. This study exemplifies the huge challenges associated with characterizing biosynthesis genes in dinoflagellates on the genomic level, regardless of the sequencing technology used. Unfortunately, sequences obtained in the study by Kubota et al. (2006) were not deposited in GenBank, preventing further analysis. Similarly, when Bachvaroff and Place (2008) tried to find other modules of a KR gene in A. carterae no other PKS modules were found within 12 kb of genomic DNA. This gene was also interspersed with numerous introns. And lastly, when Monroe and Van Dolah (2008) characterized the PKS cDNAs from K. brevis they only found transcripts that contained one catalytic module rather than multiple modules expected for a modular Type I PKS transcript. Recently a novel group of modular PKSs that diverge from the canonical (cis-AT) type architecture have been described (Nguyen 2008). These trans-AT PKSs are characterized by the absence of an integrated AT domain in each module which is complemented by free-standing acyl transferases. Phylogenetic data suggest that these trans-AT PKSs evolved independently from cis-AT PKSs. We believe this is the mode of polyketide synthesis in dinoflagellates. In addition to the unique acyl transfer mechanism, the enzymes are noteworthy for exhibiting highly aberrant architectures with modules carrying novel catalytic domains or domain orders, or having no apparent function or relation to polyketide structure. These could only be compared using bioinformatic approaches of the amino acid sequences. Approach Given the light dependency of karlotoxin production we hypothesize the starter unit for karlotoxin and amphidinol biosynthesis is glycolate that comes from photorespiration. A previous study by Murata’s group established the polyketide origin of three amphidinols, AM2, AM3 and AM4 (Houdai et al. 2001) using 13C labeled acetate. The enrichment patterns observed revealed that the carbon chain of AM17 is derived entirely from acetate units, including the pendant carbon atoms. Both C-1 and C-2 of the chain were however, unlabeled, similar to a result obtained for other amphidinols, and this portion of the chain was inferred to result from a glycolate starter unit as observed in okadaic acid and its analogs (Wright et al. 1996). Recently, glycolate was shown to be the starter unit for yessotoxin synthesis (Yamazaki et al., 2010) and adding glycolate to the culture media enhances gymnodimine production (Mountfort et al., 2006). This is currently being tested with feeding studies on K. veneficum and A. carterae. Proceedings 15th ICHA, accepted manuscript Fig. 2. Predicted domains for a plastid acyl carrier protein cDNA from K. veneficum (CCMP 2778) Fig. 3. Predicted domains for a CA-Acyl Carrier Protein Transacylase cDNA from K. veneficum (CCMP 2778). From the current annotation of the full-length cDNA libraries (>160,000) of K. veneficum and A. carterae (NSF Microbial Genome Sequencing Program grant #EF-0626678, “Dinoflagellate fulllength cDNA sequencing”) we have already identified greater than 200 genes potentially involved in fatty acid and karlotoxin (or amphidinol) synthesis. To sort through these candidate genes we have focused on the initiation of the PKS cycle looking for enzymes that might transfer glycolate to an acyl carrier protein and could also accommodate a large growing polyketide chain. We have found two candidate proteins that we believe are involved in the initiation of karlotoxin synthesis. The first (Fig. 2) is a large acyl carrier protein (ACP) (359 amino acids) that differs significantly from the smaller ACPs (~134 amino acids). This protein has a chloroplast targeting sequence. We envision the numerous smaller cytoplasmic ACPs are involved in fatty acid synthesis while the larger plastid protein receives the starter glycolate for karlotoxin synthesis. The second candidate is a malonyl carrier protein transacylase (Fig. 3) that is Proceedings 15th ICHA, accepted manuscript phylogenetically distinct from the other CoA-acyl carrier protein transacylases found in the libraries. We envision this transacylase charges the ACP with glycolate in the chloroplast. We are currently testing these hypotheses using antibodies to each of these proteins. Conclusion Only by focusing on the initiation of the PKS cycle can we sort the metabolic players involved in toxin biosynthesis from those involved in fatty acid synthesis. Even if this approach proves successful it is highly likely that many of the same players may be involved in both processes. Acknowledgements: This work was supported by the NOAA PCM grant # NA10NOS4780154. This is contribution #4 from NOAA PCM program, #4875 from the University of Maryland Center for Environmental Sciences, and contribution #145 from the Institute of Marine and Environmental Technology. References Bachvaroff T.R., Place A.R. (2008). PLoS One 3, e2929, DOI: 10.1371/journal.pone.0002929. Houdai T., Matsuoka S., Murata M. et al. (2001). Tetrahedron 57: 5551-5555. Kubota T., Iinuma Y., Kobayashi J. (2006). Biol. Pharm. Bull. 29: 1314-1318. Meng Y., Van Wagoner R.M., Misner I. et al. (2010). J. Nat. Prod. 73: 409-415. Monroe E.A. & Van Dolah F.M. (2008). Protist 159: 471-482. Mountfort D., Beuzenberg V., MacKenzie et al. (2006). Harmful Algae 5: 658-664. Nguyen T., Ishida K., Jenke-Kodama H. et al. (2008). Nat. Biotechnol. 26: 225-233. Rein K.S. & Snyder R.V. (2006). Adv. Appl. Microbiol. 59: 93-125. Wright J.L.C, Hu T., McLachlan J.L. et al. (1996). J. Am. Chem. Soc. 118: 8757-8758. Yamazaki M., Tachibana K. & Satake M. (2010). Tetrahedron 67: 877-880.