* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download More Transparency in BioAnalysis of Exocytosis: Coupling of
Biological neuron model wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Synaptogenesis wikipedia , lookup
Resting potential wikipedia , lookup
Single-unit recording wikipedia , lookup
End-plate potential wikipedia , lookup
Signal transduction wikipedia , lookup
Channelrhodopsin wikipedia , lookup
SNARE (protein) wikipedia , lookup
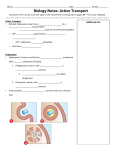
More Transparency in BioAnalysis of Exocytosis: Coupling of Electrochemistry and Fluorescence Microscopy at ITO Electrodes d Manon GUILLE-COLLIGNON Laboratoire PASTEUR, Département de Chimie, Ecole Normale Supérieure, CNRS UMR 8640, Université Pierre & Marie Curie, PARIS, FRANCE 2015, 18-19th of November, EABS Workshop, Orsay, France Exocytosis in Nerve and Humoral Transmission Phenomenon in Ph i wich i h a cellll directs di materials i l release l to the h outside id by b discharging di h i them as a membrane-bounded vesicles passing through the cell membrane. Neuron body Synaptic cleft http://lyrobossite.free.fr/Structure_II_Les synapses.htm Example p of exocytosis y in synaptic y p transmission Numerous q questions under debate Exocytosis : Numerous Questions Under Debate Transport and motion of vesicles (actin network, cytoskeleton, …) Dynamics and stability of fusion pore (flickering, ...) Nature of factors controlling fusion process (biological and physico-chemical ones) Partial or full fusion « Kiss and run » existence? Exocytosis : Regulation by Which Parameters? Biological Bi l i l Control SNAREs proteins, « key proteins » (Syntaxine, SNAP 25, VAMP, Munc 18…), Ca2+ ions, secretagogue… Physico-chemical control Membrane properties (nature of phospholipids, viscosity, membrane tension, curvature…), pH, extracellular medium composition… Main Used Analytical Tools Time resolution Electrical recordings: • Electrochemical amperometry Optical recordings: • Fluorescence microscopy Tens of µs ~ 50 ms Electrochemical Detection « Artificial synapse » configuration Ultramicroelectrode Minimization of the distance ((<1µm) µ ) Volume Femtomoles emitted within ~ picolitre ~ [mM] 7 µm Carbon fiber Faradic current [Electroactive species] Cell Signal on Noise ratio (S/N 1/r0) Detection of weak currents : 1 pA à 1 nA Response time ~ 1 ms ( r0) Study of fast biological phenomenon Detected current proportional to the concentration Advantages : Single cell level Direct measurements Selectivity offered by the potential i = 4nFDCr0 Nature of the Monitored Signals 1 spike p = 1 vesicle Cell Micropipette Chromaffin Cells 120 60 100 50 Current (pA A) Courant (pA) Microelectrode Current Couran((pA) t (pA) 140 80 60 40 Imax Imax Q Q (aire) ((area)) 40 30 t1/2 20 20 10 0 0 0 0 100 200 300 400 20 500 60 80 100 Temps (ms) Temps de Temps (s) Time (s) 40 Rising time montée Time (ms) Oxydation of catecholamines at 650 mV vs. Ag/AgCl : O HO OH OH HO Amperometric Parameters: Events frequency Charge (Q /fC) Intensity (I /pA) Half-width (t1/2 /ms) + CH3 N H H O + CH3 N H H + 2H+ + 2e- PhysicoChemistry of Exocytosis : Effect of Membrane Curvature LPC 2 µM Lysophosphatidylcholine Short time incubation AA 20 µM A hid i acid Arachidonic id LPC Fusogenic BiophysJ, 1995, 69, 922 ; ChemBioChem, 2006, 7, 1998 “Inverted cone” “Cone” AA Anti Fusogenic Membrane Curvature : Effects on Exocytosis Frequency LPC Nomb bre ments Number r ofd’évènem detecteed events 1400 1200 Fusogenic Fusogène LPC 1000 800 Control 600 400 AA 200 0 0 50 100 150 200 250 300 Time (s) Temps /s Strong effect of membrane curvature on the secretion frequency AA Anti-fusogène Anti Fusogenic Membrane Curvature : Effects on Exocytosis Dynamics 1800 40 30 AA Control LPC 1600 AA C t l Control LPC 1400 Chargee/ fC Timee /ms 1200 20 10 1000 800 600 400 200 0 t20-90 t1 t2 0 LPC favors vesicle / cell membranes fusion Quantity of released catecholamines varies and can be rised with LPC Better expansion of fusion pore? Fine regulation of exocytosis mechanism? ChemBioChem, 2006, 7, 1998 Scientific Stake to Deploy a Coupling Methodology Pi i Priming Docking Cell (diameter 10 µm) Vesicle (diameter 300 nm) Vesicle Extracellular medium Fusion Intracellular medium Cell membrane PennState Univ., USA Is it possible to achieve a detection : - of the same exocytotic event - at the same place of the cell - with two different analytical y techniques q ((optical p and electrochemical)? ) Main Used Analytical Tools 2) Total Internal Reflection Fluorescence-Microscopy py = TIRF-M 1) Electrochemical amperometry Carbon-fiber C rb n fib r ultramicroelectrode Ø = 10 µm Objective lens Cell Stimulating capillary Water Cell Glass Laser beam Principle: Excitation of fluorescent vesicles in cell by an evanescent wave of very low penetration depth (50-300 nm) Intensity (pA) Peak area Q Time (s) 2 µm Only vesicles near the plasma membrane are monitored. monitored Exocytotic events are seen as «flash» or extinction of fluorescence. Analytical Tools 1) Electrochemical amperometry 2) TIRF-Microscopy Advantages Real time detection of single events Real-time detection of single events Vesicles motion observation before fusion Quantitative information - on kinetics - on number of released molecules Drawbacks Released molecules must be electroactive “Blind” technique before fusion pore No motion information of vesicles No quantitative information Fluorescence of the vesicles is required 3 µm Specific Devices for the Coupling Required conditions: Coupled detection at the same place of the cell Detection realized at the bottom of the cell Transparent and conducting substrate Choice of ITO: Indium Tin Oxide (90% In2O3 + 10% SnO2) Electrochemical limitation: surface of ITO compromise between a suitable electrical noise cells dimensions ITO band electrodes (200 µm width) id h) Specific Devices for the Coupling Technological process 4 independent working electrodes of ITO Cellule Choice of Cells for the Coupling Choice of enterochromaffin BON cells: Required conditions: Optical detection: fluorescent probe Expressing GFP-tagged neuropeptide-Y 2 µm Releasing neurotransmitter serotonin Electrochemical detection: electroactive molecules (650 mV vs Ag/AgCl) ‐2 H+ ; ‐2 e‐ Moderate frequency of secretion: to assign to each amperometric peak the corresponding optical signal Low frequency of exocytosis 0.1 Hz Angew. Chem., 2011, 50 (22), 5081 Biophys Chem., Biophys. Chem 2012, 2012 162 162, 14 Faraday Discussion , 2013, 164, 33 Electrochimica Acta, 2014, 126, 74 Electrochimica Acta, 2014, 140, 457 Experimental Set-Up Ref Ag/AgCl ITO Cell Cells Injection capillary (stimulation) Selective S l i stimulation of a single cell (ionomycin 5 µM) Validation of the Combined Method Same trigger for the optical and amperometrical recordings. Extinction of fluorescence seen byy TIRF Example 1 Amperometric spike Fluorescence flash seen by TIRF Example 2 Amperometric spike • • ~ 4 coupled events by cell (n = 6) Different temporal resolutions : T TIRF= 100 ms, TAmperometry = 10 µs Conclusion… Use of UME/electrochemistry for unraveling physico-chemical factors controlling exocytosis Validation of a proof of conceipt : C Coupling li amperometry and d TIRF to analyze l an exocytotic i event n=66 cells (85 events) Analysis of events obtained : for n 30% of coupled events Acknowledgments UMR CNRS-ENS-UPMC 8640 « PASTEUR », » ENS ENS, PARIS Christian AMATORE Jérôme DELACOTTE Rémy FULCRAND Lihui HU Frédéric LEMAITRE Xiaoqing LIU Anne MEUNIER Damien QUINTON UMR 8192, Institut de Biologie Physico-Chimique, PARIS Marine BRETOU François DARCHEN Isabelle FANGET Ouardane JOUANNOT Université de Bordeaux 1, Institut des Sciences Moléculaires UMR 5255 5255, Pessac Stéphane ARBAULT