* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal
Cell growth wikipedia , lookup
Tissue engineering wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell encapsulation wikipedia , lookup
Lipopolysaccharide wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
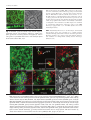
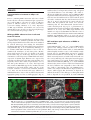
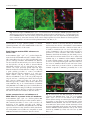
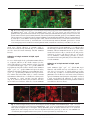
Microbiology (2004), 150, 527–538 DOI 10.1099/mic.0.26740-0 Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin Jennifer Cleary,1 Li-Ching Lai,2 Robert K. Shaw,1 Anna Straatman-Iwanowska,1 Michael S. Donnenberg,2 Gad Frankel3 and Stuart Knutton1 1 Institute of Child Health, University of Birmingham, Birmingham, UK Correspondence Stuart Knutton 2 [email protected] 3 Division of Infectious Diseases, University of Maryland, Baltimore, MD, USA Centre for Molecular Microbiology and Infection, Department of Biosciences, Imperial College, London, UK Received 28 August 2003 Revised 18 November 2003 Accepted 25 November 2003 Enteropathogenic Escherichia coli (EPEC), an important paediatric diarrhoeal pathogen, employs multiple adhesins to colonize the small bowel and produces characteristic ‘attaching and effacing’ (A/E) lesions on small intestinal enterocytes. EPEC adhesins that have been associated with A/E adhesion and intestinal colonization include bundle-forming pili (BFP), EspA filaments and intimin. BFP are involved in bacteria–bacteria interaction and microcolony formation but their role in cell adhesion remains unclear; EspA filaments are components of the EPEC type III secretion system but since they interact directly with host cells they may also function as adhesins; intimin is the well characterized intimate EPEC adhesin which binds the translocated intimin receptor, Tir. However, other uncharacterized host cell receptors have been implicated in intimin-mediated adhesion. In this study, the role of BFP, EspA filaments and intimin in EPEC adhesion to intestinal brush border cells was assessed by observing adhesion of wild-type EPEC strain E2348/69 and a set of isogenic single, double and triple mutants in bfpA, espA and eae (intimin gene) to differentiated human intestinal Caco-2 cells. E2348/69 (bfpA+ espA+ eae+) adhered rapidly (<10 min) to the brush border of Caco-2 cells and subsequently produced microcolonies and typical A/E lesions. Non-intimate brush border adhesion of double mutant strain UMD880 (bfpA+ espA” eae”) also occurred rapidly, whereas adhesion of strain UMD886 (bfpA” espA+ eae”) occurred later in the infection (>1 h) and with much lower efficiency; confocal microscopy indicated BFP and EspA-mediated adhesion, respectively. Strain UMD883 (bfpA” espA” eae+), which is unable to translocate Tir, was non-adherent although this strain was able to form intimate attachment and A/E lesions when co-cultured with strain CVD206 (bfpA+ espA+ eae”) which supplied Tir to the membrane. Single mutant strains CVD206 (bfpA+ espA+ eae”) and UMD872 (bfpA+ espA” eae+) showed adherence characteristics of strain UMD880 (bfpA+ espA” eae”), whilst triple mutant strain UMD888 (bfpA” espA” eae”) was totally non-adherent. These results support an adhesive role for BFP and EspA in initial brush border cell attachment, and in typical EPEC which express both BFP and EspA filaments suggest a predominant role for BFP; EspA filaments, however, could serve as initial attachment factors in atypical EPEC which lacks BFP. The study found no evidence for an independent host cell intimin receptor or for other adhesive factors able to support bacterial adherence. Abbreviations: A/E, attaching and effacing; BFP, bundle-forming pilus/pili; EPEC, enteropathogenic Escherichia coli ; LEE, locus of enterocyte effacement; RBC, red blood cell; SEM, scanning electron microscopy; TTSS, type III secretion system; WGA, wheat germ agglutinin. 0002-6740 G 2004 SGM Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Sat, 17 Jun 2017 05:29:40 Printed in Great Britain 527 J. Cleary and others INTRODUCTION Enteropathogenic Escherichia coli (EPEC), an important cause of infantile diarrhoea in the developing world (Nataro & Kaper, 1998), colonizes the small bowel and produces ‘attaching and effacing’ (A/E) lesions on the brush border surface of small intestinal enterocytes characterized by effacement of brush border microvilli, intimate attachment of bacteria and formation of an actin-rich pedestal beneath intimately attached bacteria (Knutton et al., 1989; Moon et al., 1983). Current evidence, derived mainly from in vitro studies using cultured epithelial cell lines, supports a model of A/E lesion formation which involves initial nonintimate bacterial adhesion, type III secretion and effector protein translocation to the host cell followed by brush border effacement, intimate bacterial attachment and pedestal formation (Frankel et al., 1998). Several adhesins have been implicated in A/E EPEC adhesion to epithelial cells including the type IV bundle-forming pilus (BFP) (Giron et al., 1991), type III secretion system (TTSS) EspA filaments (Knutton et al., 1998) and the outer-membrane adhesin, intimin (Donnenberg & Kaper, 1991). BFP, encoded on a large ~80 kb EPEC adherence factor plasmid (EAF) (Nataro et al., 1987b) present in typical EPEC strains (Kaper, 1996), have been shown to be important for EPEC pathogenicity (Bieber et al., 1998), to be responsible for bacteria–bacteria interaction and microcolony formation (Giron et al., 1991) and also for dispersal of bacteria from microcolonies (Bieber et al., 1998; Knutton et al., 1999). Several studies have also implicated BFP in initial binding of EPEC to host epithelial cells (Donnenberg et al., 1992; Giron et al., 1991; Tobe & Sasakawa, 2001, 2002) although other studies which used human intestinal organ culture suggested that BFP were not involved in initial EPEC adherence but only at a later stage to promote microcolony formation (Hicks et al., 1998). Genes encoding A/E lesion formation are localized to the locus of enterocyte effacement pathogenicity island (LEE PAI) (McDaniel et al., 1995). The LEE encodes a TTSS, several secreted translocator and effector proteins and proteins involved in intimate bacterial attachment (Frankel et al., 1998). One secreted translocator protein, EspA, forms a long filamentous extension to the TTSS needle complex (Daniell et al., 2001; Sekiya et al., 2001), whereas two other translocator proteins, EspB and EspD, are thought to provide a translocation pore in the host cell membrane, thereby allowing translocation of LEE effector proteins into the host cell cytosol (Frankel et al., 1998). Since EspA filaments form a direct interaction with the host cell during early stages of A/E lesion formation (Knutton et al., 1998), they could function as an initial EPEC attachment factor, particularly in the case of atypical EPEC which lacks EAF plasmids (Kaper, 1996). Intimin is the well characterized EPEC adhesin that promotes intimate attachment to host cells. However, to form an intimate attachment and A/E lesion formation, the 528 LEE-encoded effector protein Tir (translocated intimin receptor) has to be translocated and inserted into the host cell membrane by the TTSS (Kenny et al., 1997); the intimin–Tir interaction triggers the assembly of actin into pedestals beneath intimately attached bacteria (Campellone & Leong, 2003). Whilst Tir is a well characterized intimin receptor, additional uncharacterized host cell receptors have been implicated in intimin-mediated EPEC adhesion (Frankel et al., 2001) and recently a specific host cell protein, nucleolin, was shown to bind a specific intimin subtype, intimin c, expressed by some EPEC and enterohaemorrhagic E. coli strains (Sinclair & O’Brien, 2002). The difficulty in assessing an adhesive role of BFP, EspA filaments and intimin when they are being expressed simultaneously in wild-type EPEC prompted us to generate a set of single, double and triple mutants in bfpA, espA and eae and to examine the role of these putative adhesins individually and in pairs. Human intestinal brush border cells provide a better model of EPEC infection than do undifferentiated epithelial cells of non-intestinal origin. Hence, in this study, we examined EPEC adhesion to Caco-2 brush border cells. The study supports a role for BFP and EspA filaments in initial brush border attachment of EPEC and intimin–Tir interaction in intimate EPEC adhesion and A/E lesion formation. The study found no evidence for other adhesins able to promote EPEC adhesion. METHODS Bacterial strains. The strains and plasmids used in the study are shown in Table 1. All mutants are derivatives of EPEC serotype O127 : H6 strain E2348/69 (Levine et al., 1978). The following mutant strains have been described previously: eae deletion mutant strain CVD206, which does not produce intimin (Donnenberg & Kaper, 1991); espA deletion mutant strain UMD872, which does not produce EspA (Kenny et al., 1996); and bfpA mutant strain UMD901, which has a substitution of serine for cysteine at amino acid 129 that renders the bundlin pilin protein unstable and rapidly degraded and therefore does not produce BFP (Zhang & Donnenberg, 1996). The suicide vectors originally used to generate these mutants were used to make the following by allelic exchange: UMD880 is an espA eae double mutant; UMD883 is a bfpA espA double mutant; UMD886 is a bfpA eae double mutant; and UMD888 is a bfpA espA eae triple mutant. Plasmids pRPA100, pMSD2 and pCVD438, used to transform bfp, espA and eae, respectively, have been described previously (Anantha et al., 2000; Donnenberg & Kaper, 1991; Kenny et al., 1996). Bacteria were routinely grown overnight in Luria broth (LB) and then subcultured (1 : 100) in tissue culture media for cell adhesion studies. Bacterial motility assays were performed in plastic vials in LB and Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 0?3 % agar and read after 18 h incubation at 37 uC. Infection of HEp-2 cells. Adhesion to HEp-2 cells was carried out according to the method of Cravioto et al. (1979). Subconfluent cell cultures on glass coverslips were washed and incubated with bacteria [10 ml overnight LB culture per millilitre of tissue culture medium (DMEM/5 % fetal calf serum)] for 3 h at 37 uC. After thorough washing to remove non-adhering bacteria, cells were either fixed in methanol and stained with Giemsa to assess the pattern of cell Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Sat, 17 Jun 2017 05:29:40 Microbiology 150 EPEC adhesins Table 1. Strains and plasmids used in the study Illustrated are strains, their bfpA espA eae genotypes, their phenotypes in HEp-2 and Caco-2 cell assays and their motility. HEp-2 cell adhesion, 2 to +++++; LA, localized adherence; FAS, fluorescence actin staining test (+ indicates A/E lesion formation). Caco-2 cell adhesion, mean±SD [relative to wild-type E2348/69 (100 %)]. Strains Genotype bfpA E2348/69 UMD880 UMD886 UMD883 UMD888 UMD901 UMD872 CVD206 Plasmids pRPA100 pMSD2 pCVD438 espA + + 2 2 2 2 + + + 2 + 2 2 + 2 + Phenotype eae + 2 2 + 2 + + 2 HEp-2 cells Reference or source + + +/2 + + + + + Levine et al. (1978) This study This study This study This study Zhang & Donnenberg (1996) Kenny et al. (1996) Donnenberg & Kaper (1991) Caco-2 cells Adhesion LA FAS Adhesion LA FAS ++++ ++++ + 2 2 + ++++ ++++ + + 2 2 2 2 + + + 2 2 2 2 + 2 2 100±4 110±4 8±1 0±0 0±0 7±1?5 72±4 59±3?5 + + 2 2 2 2 + + + 2 2 2 2 + 2 2 Cloned bfpA Cloned espA Cloned eae Anantha et al. (2000) Kenny et al. (1996) Donnenberg & Kaper (1991) adhesion or fixed in 4 % formalin and A/E lesion formation assessed using the fluorescent actin staining test (Knutton et al., 1989). Semiquantitative assessment of adhesion was made by counting 100 cells in representative microscope fields and determining the percentage of cells with adherent bacteria. Adhesion was scored from 2 (nonadherent) to +++++ (80–100 % cells with adherent bacteria). Infection of Caco-2 cells. Caco-2 intestinal cells were grown in DMEM/20 % fetal calf serum on 24 mm diameter Transwell permeable culture supports for 12 days to ensure differentiation of the brush border. EPEC infections were performed in HEPES-buffered Modified Eagle’s Medium (MEM)/2 % fetal calf serum. Cell monolayers were infected with an overnight LB culture of EPEC [10 ml broth culture (ml culture medium)21] for up to 6 h at 37 uC; for long infections, the medium was changed after 3 h. In co-culture experiments, 5 ml broth culture (ml medium)21 of each strain was used. At the end of the infection period, cells were washed three times in PBS for 1 min on an orbital shaker and fixed in either 4 % formalin (for immunofluorescence studies) or 3 % buffered glutaraldehyde [for scanning electron microscopy (SEM)]. In some experiments, a gentle washing procedure was used. In this case cells were washed three times for 20 s with gentle rocking by hand. Quantitative assessment of adhesion was made from two separate infections by counting numbers of bacteria in three representative microscope fields taken with a 636 objective lens. Adhesion (mean number of bacteria per field±SD) is expressed relative to that of E2348/69 (100 %). Infection of human red blood cell (RBC) monolayers. RBC monolayers were produced as described previously (Knutton et al., 2002). EPEC infections were performed in HEPES-buffered DMEM and cell monolayers infected with an overnight LB culture of EPEC [10 ml broth culture (ml culture medium)21] for 3 h at 37 uC. After three washes in PBS, monolayers were fixed in 4 % formalin. Infection of human intestinal mucosa. Normal paediatric duo- denal mucosal biopsies taken with informed consent were transported to the laboratory in ice-cold transport medium as described previously (Knutton et al., 1987) and used immediately. Bacteria http://mic.sgmjournals.org Motility grown for 3 h in DMEM express LEE genes and are ‘primed’ ready to produce A/E lesions (Collington et al., 1998). Primed bacteria were used to infect mucosal biopsies. Biopsies were placed in Bijoux bottles with 1?5 ml primed bacterial culture and 1?5 ml fresh culture medium (DMEM : NCTC135/10 % FCS) and the bottles placed on a rotary mixer inside an incubator for 1?5 h at 37 uC. Biopsies were washed three times in warm PBS on the rotary mixer and fixed in 3 % glutaraldehyde. Antibodies and fluorescent stains. Polyclonal rabbit or mouse antibodies to BFP (Knutton et al., 1999), EspA (Knutton et al., 1998), intimin (Batchelor et al., 1999), Tir (Hartland et al., 1999) and H6 flagellin (Yona-Nadler et al., 2003) were used for immunofluorescence staining in conjunction with Alexa488 (green) and Alexa594 (red) goat anti-rabbit IgG or goat anti-mouse IgG secondary antibody conjugates (Molecular Probes). In addition, bacteria were stained with propidium iodide (red) or DAPI (blue) (Molecular Probes), cell actin with fluorescein (green) or rhodamine (red) conjugated phalloidin (Sigma) and RBCs and the brush border membrane with rhodamine-conjugated wheat germ agglutinin (WGA) (Sigma). Immunofluorescence microscopy. To stain Tir and cytoskeletal actin, Caco-2 cells were permeabilized with PBS containing 0?1 % Triton X-100 for 5 min and washed three times in PBS prior to immunostaining. All antibody dilutions and immune reactions were carried out in PBS containing 0?2 % bovine serum albumin (PBS/BSA). Formalin-fixed, permeabilized and washed cells were incubated with antiserum (1 : 100) in PBS/BSA for 45 min at room temperature. After three 5 min washes in PBS, samples were stained with either Alexa488- or Alexa594-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (Molecular Probes) diluted 1 : 100 in PBS/ BSA for 45 min. Where appropriate, samples were simultaneously stained with propidium iodide to stain bacteria and/or phalloidin to stain cytoskeletal actin- or rhodamine-conjugated WGA to stain the brush border membrane. RBC samples were stained with rhodamineconjugated WGA to stain the RBC membrane and with DAPI to stain bacteria. Preparations were washed a further three times in Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Sat, 17 Jun 2017 05:29:40 529 J. Cleary and others PBS and mounted in glycerol/PBS. HEp-2 and Caco-2 cell preparations were examined by confocal microscopy using a Leica TCS SP2 Spectral Confocal Microscope, equipped with an Argon (488 nm) and two Helium/Neon (543 nm, 633 nm) lasers and 406 or 636 PL APO objectives. Confocal illustrations show either image sections or image projections through the brush border region of the cell. In triple-stained specimens using Alexa488, Alexa594 and propidium iodide, the propidium iodide emission was artificially coloured blue to distinguish it from Alexa594 (red). RBC samples were examined by conventional epifluorescence microscopy using a Leica DMR microscope equipped with a 636 PL APO objective and a Leica DC200 digital camera. Fig. 1. Horizontal xy (a) and vertical xz (b) confocal images of uninfected Caco-2 cell monolayers stained for cellular actin (FITC, green) and a scanning electron micrograph (c). Caco-2 cells grown on permeable filters have a well developed apical brush border surface. Bars, 5 mm. SEM. Glutaraldehyde-fixed Caco-2 cell monolayers and intestinal mucosal biopsy tissue were post-fixed in 1 % osmium tetroxide, dehydrated through graded alcohol solutions and critical-point-dried. Mounted specimens were sputter-coated with platinum (Polaron) and examined in a Philips XL30 scanning electron microscope (Knutton, 1995). Fig. 2. Confocal (a–g) and SEM (h) images of Caco-2 cell monolayers infected with E2348/69 (bfpA+ espA+ eae+). Within 10 min bacteria are seen adhering to the brush border (phalloidin–FITC, green) as single bacteria (propidium iodide, red) (a); adherent bacteria express BFP (Alexa594, red), EspA filaments (Alexa488, green) and intimin (Alexa488, green, inset) (b). BFP fibrils (Alexa488, green) appear to promote attachment of bacteria (propidium iodide, coloured blue) to the brush border (phalloidin–TRITC, red) and also bacteria–bacteria aggregation (c). After 3 h, horizontal (d) and vertical (e) confocal images following BFP (Alexa488, green), bacteria (propidium iodide, blue) and actin (phalloidin–TRITC, red) staining revealed adherent three-dimensional microcolonies of bacteria connected by BFP. After 6 h, horizontal (f) and vertical (g) confocal images following staining for bacteria (propidium iodide, red) and actin (phalloidin–FITC, green) showed A/E lesion formation (actin accretion beneath bacteria) (f, g, arrows); bacterial microcolonies were no longer present. A/E lesion formation (h, arrow) and an absence of microcolonies was also seen by SEM (h). Bars, (a–g) 5 mm; (h) 1 mm. 530 Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Sat, 17 Jun 2017 05:29:40 Microbiology 150 EPEC adhesins RESULTS Characterization of mutants in HEp-2 cell assays Prior to examining EPEC interaction with Caco-2 brush border cells, the collection of mutants in bfpA, espA and eae was tested in HEp-2 cell assays for extent of adhesion, pattern of adhesion (Nataro et al., 1987a) and fluorescent actin staining (Knutton et al., 1989). Expression of BFP, EspA filaments and intimin were assessed by immunofluorescence. In each case the observed phenotype was consistent with the genotype (Table 1). Wild-type EPEC adhere to Caco-2 cells and form A/E lesions Caco-2 cells grown on permeable filters for 12 days produce a polarized sheet of columnar epithelial cells with a well developed apical brush border surface that can be visualized by confocal microscopy after staining the microvillous membrane with WGA or the microvillous actin cytoskeleton with phalloidin (Fig. 1a, b) or visualized by SEM (Fig. 1c). To examine initial EPEC attachment to Caco-2 cells, we examined cell monolayers at early time points (10 min) when single bacteria were first seen bound to the brush border surface (Fig. 2a). At this stage, fluorescence staining of E2348/69 revealed expression of BFP fibrils and surface expression of intimin; EspA filaments were expressed by some but not all bacteria at this time point and they were frequently shorter than mature EspA filaments seen at later time points (Knutton et al., 1998). The longer and more numerous BFP fibrils appeared to mediate bacterial attachment to the brush border surface (Fig. 2b). Actin accretion, indicative of A/E lesion formation (Knutton et al., 1989), was not observed at this stage. Adhesion of individual bacteria to Caco-2 cells was followed by bacterial aggregation giving rise first to small aggregates (Fig. 2c) and later after 3 h to larger microcolonies (Fig. 2d) typical of localized adherence seen with HEp-2 cells (Table 1). All bacteria within microcolonies expressed BFP and BFP appeared to mediate bacteria–bacteria aggregation (Fig. 2d, e); actin accretion beneath bacteria in contact with the apical cell surface indicated that they had formed A/E lesions (Fig. 2f, g). By 6 h only bacteria in contact with the cell surface that had produced A/E lesions were observed, indicating that the three-dimensional bacterial microcolonies had dispersed (Fig. 2g, h), a phenomenon we demonstrated previously in E2348/69-infected HEp-2 cells (Knutton et al., 1999). Since BFP, EspA filaments and intimin were expressed when EPEC first adhered to Caco-2 cells, we examined double deletion mutant strains UMD880, UMD886 and UMD883 (Table 1) to assess the role in adhesion of BFP, EspA filaments and intimin independently. BFP mediates rapid adherence of EPEC to Caco-2 cells Strain UMD880 (bfpA+ espA2 eae2) expressed BFP bundles typical of the wild-type strain but there were no EspA filaments and no surface intimin (Table 1). Strain UMD880 adhered rapidly (10 min) to Caco-2 cells, initially as single bacteria (Fig. 3a) and BFP fibrils appeared to promote brush border attachment of bacteria (Fig. 3b, c). BFP-like fibrils connecting bacteria to brush border microvilli were also seen by SEM (Fig. 3d). Subsequent bacterial aggregation resulted after 3 h in typical localized microcolonies with bacteria interlinked by BFP (Fig. 3e, f ). Strain UMD880 did not induce actin accretion or A/E lesion formation but the level of adhesion was comparable to that of the wildtype (Table 1). Interestingly, after 6 h UMD880 microcolonies were still present and had not dispersed (data not shown), which suggests that the kinetics of dispersal are different from those of the wild-type or that signals which lead to dispersal are not produced by strain UMD880. Fig. 3. Confocal (a–c, e, f) and SEM (d) images of UMD880 (bfpA+ espA” eae”) infected Caco-2 cell monolayers. Within 10 min individual bacteria (propidium iodide, red) adhere to the brush border (stained for actin, phalloidin–FITC, green) (a). BFP fibrils (Alexa488, green) seen in horizontal (b, arrow) and vertical (c, arrow) confocal images appear to promote adhesion of UMD880 to the brush border (phalloidin–TRITC, red). BFP-like fibrils connecting bacteria to brush border microvilli were also seen by SEM (d, arrows). After 3 h, horizontal (e) and vertical (f) confocal images following BFP (Alexa488, green), bacteria (propidium iodide, coloured blue) and actin (phalloidin–TRITC, red) staining revealed adherent three-dimensional microcolonies of bacteria interlinked by BFP. Bars, (a–c, e, f ) 5 mm; (d) 1 mm. http://mic.sgmjournals.org Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Sat, 17 Jun 2017 05:29:40 531 J. Cleary and others Fig. 4. Confocal images of UMD886 (bfpA” espA+ eae”) infected Caco-2 cell monolayers. UMD886 (propidium iodide, red) adhered poorly to the Caco-2 cell brush border (WGA–FITC, green) even after 3 h (a). However, an ~10-fold increase in the level of UMD886 adhesion was seen following a gentle wash procedure (b). In each case EspA filaments (Alexa488, green) seen in horizontal (c, arrow) and vertical (d, arrow) confocal images appeared to promote adhesion of bacteria (propidium iodide, coloured blue) to the cell brush border (phalloidin–TRITC, red). Bars, 5 mm. Transformation of EspA (plasmid pMSD2) or intimin (plasmid pCVD438) into strain UMD880 did not alter the adherence characteristics of this strain. EspA filaments mediate EPEC adherence to Caco-2 cells Strain UMD886 (bfpA2 espA+ eae2) produced EspA filaments but lacked both BFP and surface intimin (Table 1). This strain adhered to Caco-2 cells but inefficiently compared to wild-type E2348/69 and UMD880. No adhesion was detected after 10 min and only a low level of adhesion was detected after 3 h (Fig. 4a, Table 1); bacteria adhered individually and there was no aggregation into microcolonies. We noted previously greatly improved EspA filament-mediated bacterial adhesion to HEp-2 cells using a gentle washing procedure to remove non-adherent bacteria following infection (Knutton et al., 1998). In a similar manner, using the gentle washing procedure, adhesion of UMD886 to Caco-2 cells was greatly improved (Fig. 4b) and was ~10-fold greater than when using the more vigorous washing procedure. In each case EspA filaments appeared to mediate brush border adhesion of strain UMD886 (Fig. 4c, d). Transformation of UMD886 with pRPA100 restored BFP expression, localized adhesion, microcolony formation and good adhesion; transformation with pCVD438 did not affect the overall level of adherence but conferred A/E lesion formation (data not shown). Intimin promotes Caco-2 cell adherence of EPEC when Tir is present in the cell membrane Strain UMD883 (bfpA2 espA2 eae+) did not produce BFP or EspA filaments but did express surface intimin (Table 1). This strain did not adhere to Caco-2 cells even after 6 h, irrespective of the washing procedure used (Table 1). Transformation of UMD883 with pRPA100 restored BFP expression and microcolony formation whilst transformation with pMSD2 restored adherence comparable to strain UMD886 and A/E lesion formation (data not shown). 532 Intimin is known to bind Tir following its translocation and insertion into the host cell membrane; strain UMD883 lacks EspA filaments and thus a functional TTSS and so is unable to translocate Tir. To assess the adhesive properties of UMD883 to cells possessing translocated Tir, we performed co-culture infections with strain CVD206 or UMD886, which lack the intimin gene but which possess a functional TTSS and can translocate Tir. In co-culture with CVD206 (Fig. 5) or UMD886, strain UMD883, identified by surface intimin staining, was able to adhere intimately to Caco-2 cells and produced typical A/E lesions. However, the extent of UMD883 adhesion and A/E lesion formation was much more pronounced when co-cultured with CVD206 compared to UMD886, presumably due to the Fig. 5. Confocal (a–c) and SEM (d) images of Caco-2 cells co-infected with UMD883 (bfpA” espA” eae+) and CVD206 (bfpA+ espA+ eae”) for 3 h and stained only for UMD883 bacteria (intimin staining). In horizontal (a) and vertical (b) confocal images of cells stained for actin (phalloidin–FITC, green), UMD883 bacteria (Alexa594, red) were now adherent and produced A/E lesions (actin accretion beneath bacteria) (b, arrow). Focused Tir (Alexa488, green) was also seen beneath intimately attached UMD883 bacteria (Alexa594, red) (c). A/E lesions produced by UMD883 (d, arrow) were also seen by SEM. Bars, 1 mm. Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Sat, 17 Jun 2017 05:29:40 Microbiology 150 EPEC adhesins Fig. 6. Confocal images showing Caco-2 cell monolayers infected with single mutant strains CVD206 (bfpA+ espA+ eae”) (a), UMD872 (bfpA+ espA” eae+) (b) and UMD901 (bfpA” espA+ eae+) (c). Caco-2 cells were stained for actin (phalloidin–FITC, green) and bacteria stained with propidium iodide (red). CVD206 adhered rapidly (10 min) to Caco-2 cells (a) and adherent bacteria expressed BFP (Alexa594, red) and EspA filaments (Alexa488, green) (a, inset). UMD872 similarly induced rapid Caco-2 cell adhesion (b) and adherent UMD872 produced BFP fibrils (Alexa594, red) but no EspA filaments (b, inset). By 6 h, some UMD901 bacteria (propidium iodide, red) had formed A/E lesions (c, arrow). Other adherent bacteria had not induced actin accretion (c, arrowhead) and in such bacteria EspA filaments (Alexa488, green) seen in vertical confocal images appeared to mediate brush border (phalloidin–TRITC, red) adhesion of bacteria (c, inset). Bar, 5 mm; inset 1 mm. much more efficient adhesion of CVD206 (Table 1). Focused Tir translocated by CVD206 (Fig. 5c) or UMD886 was also detected beneath intimately attached UMD883 bacteria. Adhesion of single mutants in bfpA, espA and eae To assess which might be the predominant EPEC adhesin, we compared adhesion of the double mutants just described with adhesion of single mutants in bfpA, espA and eae. Strain CVD206 (bfpA+ espA+ eae2) produced BFP and EspA filaments but lacked surface intimin; strain UMD872 (bfpA+ espA2 eae+) produced BFP and expressed surface intimin but lacked EspA filaments; strain UMD901 (bfpA2 espA+ eae+) produced EspA filaments and expressed surface intimin but lacked BFP (Table 1). Strains CVD206 and UMD872 exhibited Caco-2 cell adherence characteristic of UMD880, i.e. rapid (10 min) bacterial adhesion (Fig. 6a, b) followed by microcolony formation but there was no A/E lesion formation. Adherence of strain UMD901 was characteristic of strain UMD886, i.e. no adhesion after 10 min and poor adhesion after 3 h. However, unlike strain UMD886, adherent UMD901 bacteria do possess a functional TTSS and were able to translocate Tir and produce A/E lesions on Caco-2 cells after 6 h (Fig. 6c). As was the case for UMD886, a mild washing procedure to remove non-adherent bacteria significantly increased the adherence of strain UMD901. Adhesion of a triple mutant in bfpA, espA and eae Strain UMD888 (bfpA2 espA2 eae2) lacked BFP, EspA filaments and intimin and was non-adherent to Caco-2 cells after 6 h irrespective of which washing procedure was used (Fig. 7d, Table 1). Transformation with plasmid pRPA100 restored adherence and microcolony formation typical of UMD880; transformation with pMSD2 conferred a weak, non-localized cell adherence to cells typical of UMD886; UMD888 transformed with pCVD438 remained non-adherent (data not shown). Fig. 7. Confocal images showing Caco-2 cell monolayers infected with EPEC strain E2348/69 (bfpA+ espA+ eae+) for 10 min (a), 3 h (b) and 6 h (c) and with strain UMD888 (bfpA” espA” eae”) for 6 h (d). Bacteria were stained with propidium iodide (red) and flagella with an anti-flagellin antibody (Alexa488, green). All bacteria attached after 10 min possessed flagella (a) but with increasing time fewer and fewer flagellate bacteria were present (b, c). After 6 h, strain UMD888 was non-adherent (d) but flagellate bacteria were present in culture supernatants (d, inset). Bars, 5 mm. http://mic.sgmjournals.org Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Sat, 17 Jun 2017 05:29:40 533 J. Cleary and others Fig. 8. Fluorescence images showing EPEC adhesion to RBC monolayers. Bacteria were stained with DAPI (blue) and the RBC membrane with WGA–TRITC (red). Strains UMD886 (bfpA” espA+ eae”) (d), UMD901 (bfpA” espA+ eae+) (f) and CVD206 (bfpA+ espA+ eae”) (h) adhered to RBC monolayers in similar numbers to wild-type E2348/69 (bfpA+ espA+ eae+) (a), whereas strains UMD880 (bfpA+ espA” eae”) (b), UMD883 (bfpA” espA” eae+) (c), UMD888 (bfpA” espA” eae”) (e) and UMD872 (bfpA+ espA” eae+) (g) did not adhere. Bar, 5 mm. Flagella and EPEC adhesion to Caco-2 cells Flagella were recently implicated in EPEC adhesion to epithelial cells (Giron et al., 2002). Since strain E2348/69 produced flagella which might be contributing to adhesion, we examined motility and flagella expression of all the strains; all the strains were motile when grown in LB and in the DMEM used for cell adhesion assays (Table 1) and in each strain flagella expression was detected by immunofluorescence using an anti-flagellin antibody. Flagella expression was also examined during cell adhesion. Virtually all E2348/69 bacteria attaching initially to Caco-2 cells expressed flagella (Fig. 7a) but as bacteria formed microcolonies and produced A/E lesions (3 h) the percentage of flagellated bacteria was greatly reduced (Fig. 7b); after 6 h few flagella were observed (Fig. 7c). A similar decrease in the number of flagellate bacteria following initial attachment was seen with the other adherent strains (UMD880, UMD872, UMD901, CVD206). Strains UMD883 and UMD888 (Fig. 7d) were non-adherent to Caco-2 cells but the presence of flagellate bacteria was confirmed by immunofluorescence of bacteria present in the culture medium (Fig. 7d, inset). EPEC adhesion to RBC monolayers In recent studies we demonstrated EPEC adhesion to RBC monolayers, initially by EspA filaments and subsequently with strains able to translocate Tir, by intimin–Tir intimate 534 interaction (Shaw et al., 2001, 2002). In this study we also screened the collection of mutants in bfpA, espA and eae for their ability to adhere to RBC monolayers. As reported previously, wild-type E2348/69 adhered to RBC monolayers (Fig. 8a) and induced a high level (~80 %) of haemolysis (Shaw et al., 2001). Similar levels of RBC adhesion (and haemolysis) were produced by strains UMD886 (Fig. 8d), UMD901 (Fig. 8f ) and CVD206 (Fig. 8h), whereas strains UMD880 (Fig. 8b), UMD883 (Fig. 8c), UMD888 (Fig. 8e) and UMD872 (Fig. 8g) did not adhere to RBC monolayers and did not induce haemolysis. Thus, adhesion to RBCs was independent of BFP expression but correlated with expression of EspA filaments; under the assay conditions used, adhesion of the four positive strains was shown to be mediated by EspA filaments (data not shown). EPEC adhesion to human intestinal mucosa Studies using human intestinal organ culture suggested that BFP was not involved in initial EPEC adherence but only at a later stage to promote microcolony formation (Hicks et al., 1998). We showed previously that wild-type EPEC strain E2348/69 will colonize the surface of cultured human intestinal mucosa and produce typical A/E lesions after a 6–8 h incubation (Knutton et al., 1987). However, in these studies we did not attempt to examine early stages of adhesion. Here we examined early stages of E2348/69 adhesion to human intestinal mucosa (Fig. 9b) in comparison with CVD206 (bfpA+ espA+ eae2), which cannot produce A/E lesions but which does produce BFP and Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Sat, 17 Jun 2017 05:29:40 Microbiology 150 EPEC adhesins EspA filaments and may therefore be able to undergo initial mucosal attachment if BFP and/or EspA filaments are adhesins (Fig. 9c). After a shorter 1?5 h incubation with E2348/69, SEM revealed adhesion of bacterial microcolonies to mucosal cells in which brush border microvilli had become perturbed and elongated. Some bacteria appeared deeply embedded in the brush border (Fig. 9b), typical of bacteria that have formed intimate attachment and A/E lesions. CVD206 adhered in similar small microcolonies and induced elongation of brush border microvilli but did not appear to be intimately attached to the mucosal surface (Fig. 9c). Uninfected control tissue had a uniformly smooth appearance and lacked any adherent bacteria (Fig. 9a). DISCUSSION Intestinal colonization and A/E lesion formation by EPEC is a complex multi-stage process and several different well characterized and putative adhesive factors involved in this process have been described including BFP, EspA filaments and intimin. Observation of the interaction with Caco-2 cells of wild-type EPEC strain E2348/69 and a set of defined bfpA, espA and eae mutants and transformants supports a role for BFP and EspA filaments in initial brush border cell attachment of EPEC whereas intimin was required for intimate EPEC adhesion and A/E lesion formation providing that Tir had been translocated to the host cell. Several studies have implicated BFP in initial binding of EPEC to host cells. The initial studies of Giron et al. (1991) demonstrated a significant reduction in localized adherence of EPEC in the presence of BFP antibodies; residual adherence can now be explained by EspA filament and intimin–Tir adherence. Tobe & Sasakawa (2001) implicated BFP in host cell attachment by demonstrating preferential binding of BFP, producing EPEC to the Caco-2 cell surface rather than to existing EPEC microcolonies formed during an earlier infection. These authors also concluded that differential EPEC adherence to cells of different species origin was due to BFP (Tobe & Sasakawa, 2002). This present study, which examined EPEC strains at an early stage when they first adhere to cells, also supports such a cell adhesive role for BFP. Early rapid adhesion of individual bacteria correlated with the expression of BFP and, by immunofluorescence, BFP fibrils appeared to connect bacteria to the brush border surface. Subsequently, individual bacteria aggregated to produce small and then larger microcolonies with BFP appearing to link bacteria in the aggregates. Thus, these morphological data also support a direct role for BFP in initial cell attachment in addition to their recognized role in microcolony formation and dispersion. An adhesive role for BFP implies a specific host cell BFP receptor. Numerous candidate BFP receptors have been proposed, including a variety of oligosaccharides and, most recently, the phospholipid phosphatidylethanolamine (PE) (Nougayrede et al., 2003). This study also confirmed our previous conclusion that EPEC adhesion to RBCs is independent of BFP (Shaw et al., 2001) and indicates a BFP receptor that is lacking on the RBC surface. A PE receptor would be consistent with these RBC data because in the erythrocyte membrane PE is predominantly localized on the cytosolic side of the membrane. A study by Hicks et al. (1998) employing in vitro intestinal organ culture to examine EPEC adhesion to human small intestinal mucosa concluded that BFP was not involved in initial EPEC adherence but only at a later stage to promote microcolony formation. This conclusion was based on the fact that strain JPN15, which lacks the large plasmid encoding BFP but expresses EspA and intimin, adhered to cultured paediatric small intestinal mucosa in twodimensional microcolonies and produced A/E lesions whereas strain CVD206 (bfpA+ espA+ eae2) did not adhere. We also demonstrated previously A/E adhesion of a plasmid-cured derivative of E2348/69 to human intestinal Fig. 9. SEM images of uninfected small intestinal mucosa (a) and following a 1?5 h infection with primed E2348/69 (bfpA+ espA+ eae+) (b) and CVD206 (bfpA+ espA+ eae”) (c) bacteria. Uninfected mucosa had a smooth mucosal surface and no adherent bacteria were present (a). E2348/69 adhered in microcolonies to mucosal cells in which the brush border had been perturbed and microvilli elongated. Some bacteria appeared deeply embedded in the brush border, typical of those that form intimate attachment and A/E lesions (b). CVD206 similarly adhered in microcolonies and induced elongation of brush border microvilli but did not appear intimately attached to the mucosal surface (c). Bars, (a) 10 mm; (b, c) 5 mm. http://mic.sgmjournals.org Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Sat, 17 Jun 2017 05:29:40 535 J. Cleary and others mucosa and noted that the extent of adhesion was highly attenuated compared to that of the wild-type (Knutton et al., 1987). This could now be explained by a lack of BFP-mediated initial attachment with initial attachment of the plasmid-cured strain being promoted by the much less efficient EspA filaments. In this study we examined early stages of EPEC adhesion to human intestinal mucosa and, in contrast to Hicks et al. (1998), we did observe adhesion of strain CVD206. CVD206, unlike E2348/69, lacks intimin and so cannot form an intimin–Tir intimate attachment and A/E lesions but it does express BFP and EspA filaments which could be promoting non-intimate adhesion. Although the CVD206 adhesins involved were not identified, the observation that CVD206 did adhere does cast doubt on the conclusions of Hicks et al. that BFP does not promote initial mucosal attachment of EPEC. Failure to detect mucosal adhesion of CVD206 by Hicks et al. may have been due to differences in the adhesion assay protocols used and/or to poor BFP expression by the CVD206 strain used. It was particularly noticeable in the study by Hicks et al. that in a 3 h HEp-2 cell adhesion assay they only demonstrated adhesion of individual CVD206 bacteria to the HEp-2 cell surface, whereas others had observed adhesion of large bacterial microcolonies by 3 h (Donnenberg & Kaper, 1991; Knutton et al., 1999). EspA filaments are a component of the EPEC TTSS whose primary function is the delivery of virulence proteins into host cells (Daniell et al., 2001). However, since EspA filaments interact with host cells during the early stages of A/E lesion formation, it has been proposed that they may also function as adhesins. We showed previously that EspA filaments promoted attachment of strains lacking BFP to epithelial cells and to RBCs (Knutton et al., 1998; Shaw et al., 2001) and similar results supporting a role of EspA filaments as initial attachment factors were demonstrated with Shiga toxin-producing E. coli (Ebel et al., 1998) which also lacks BFP. This present study also supports an adhesive role for EspA filaments in EPEC adhesion to brush border cells since adhesion correlated with EspA filament expression and, by immunofluorescence, EspA filaments appeared to connect bacteria to the brush border surface. However, adhesion was much less efficient than with strains expressing BFP. The extent of EspA-filament-mediated adhesion was dependent on the washing procedure used, suggesting that EspA-filament-mediated adhesion is weak compared to BFP and intimin–Tir-mediated adhesion where the washing procedure had no effect on levels of adhesion. Compared to BFP, the less efficient EspA-filament-mediated adhesion probably reflects the small number of EspA filaments produced (~12 EspA filaments per bacterium) (Daniell et al., 2001) and the nature of their interaction with host cells which is currently unknown. EPEC have been divided into typical EPEC which possess EAF plasmids and BFP and atypical EPEC which lack EAF plasmids and BFP (Kaper, 1996). The use of EspA filaments as initial attachment factors could explain why atypical EPEC is still able to colonize the gut and produce A/E lesions but, 536 due to a lack of BFP, is a much less efficient colonizer than typical EPEC (Levine et al., 1985). Intimin expressed on the bacterial surface binds Tir following its translocation and insertion into the host cell membrane; intimin–Tir interaction produces the characteristic intimate EPEC attachment and triggers subsequent A/E lesion formation (Campellone & Leong, 2003; Frankel et al., 1998). Strain UMD883 expressing only intimin did not adhere to Caco-2 cells although it was able to bind and form an intimate attachment and A/E lesions following translocation of Tir into the Caco-2 cell membrane. The adhesive role of intimin binding to translocated Tir is well established (Kenny et al., 1997). However, there is also a considerable body of evidence for a host cell intimin receptor, not least of which is the binding of the cell-binding domain of intimin to epithelial cells in the absence of Tir (Frankel et al., 2001) and the recent demonstration, in the case of E. coli O157 : H7, of a host cell surface protein, nucleolin, which specifically binds intimin c expressed by this serotype (Sinclair & O’Brien, 2002). Although available evidence supports the presence of host cell intimin receptors, the inability of strain UMD883 to adhere to Caco2 and HEp-2 cells in the absence of Tir clearly indicates the lack of a receptor able to support bacterial adhesion. The present study examined three particular EPEC adhesive factors. However, other putative EPEC adhesive factors have been proposed, including other fimbrial antigens (Giron et al., 1993), Efa1/LifA (Badea et al., 2003) and flagella (Giron et al., 2002). Other than type 1 fimbriae, which have been demonstrated not to be involved in epithelial cell adhesion, strain E2348/69 has not been reported to produce other recognized fimbriae (Elliott & Kaper, 1997). Efa1/LifA, a protein unique to EPEC (Klapproth et al., 2000) and other A/E pathogens, appears to have cell-binding activity and might contribute to adhesion in some manner. However, the lack of adhesion of UMD888 (bfpA2 espA2 eae2) implies the absence of other adhesive factors sufficient to promote adhesion of EPEC to intestinal brush border cells. A direct role for flagella in EPEC adhesion was suggested recently (Giron et al., 2002). We thought previously that adherent EPEC did not express flagella because we did not see flagella by SEM. However, it is now clear from the immunofluorescence staining performed as part of this study that adherent EPEC do possess flagella which could therefore play a role in adhesion. Nevertheless, this study found no direct evidence for an adhesive role for flagella since strains UMD883 (bfpA2 espA2 eae+) and UMD888 (bfpA2 espA2 eae2) produced flagella and yet were totally non-adherent. However, it is clear that EPEC flagella can interact with host cells and stimulate host immune responses (Zhou et al., 2003) and thus a contribution to adhesion cannot be ruled out although this study indicates that flagella, in the absence of other adhesins, cannot by themselves support bacterial adhesion. In summary, the results of this study, using defined EPEC Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Sat, 17 Jun 2017 05:29:40 Microbiology 150 EPEC adhesins E2348/69 mutants and Caco-2 brush border cells, are consistent with data obtained previously using undifferentiated epithelial cells of non-intestinal origin and indicate that BFP, EspA filaments and, in a Tir-dependent manner, intimin can each support bacterial adhesion to intestinal epithelial cells; no evidence was found for other adhesins which could support EPEC adhesion. BFP appeared to be the predominant initial attachment factor but, in the absence of BFP, EspA filaments were able to promote a less efficient initial bacterial attachment. Intimin functioned as an adhesin in the presence of translocated Tir but we found no evidence for an independent host cell intimin receptor capable of promoting EPEC adhesion. induction of actin rearrangements depend on filamentous EspAcontaining surface appendages. Mol Microbiol 30, 147–161. Elliott, S. J. & Kaper, J. B. (1997). Role of type 1 fimbriae in EPEC infections. Microb Pathog 23, 113–118. Frankel, G., Phillips, A. D., Rosenshine, I., Dougan, G., Kaper, J. B. & Knutton, S. (1998). Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol Microbiol 30, 911–921. Frankel, G., Phillips, A. D., Trabulsi, L. R., Knutton, S., Dougan, G. & Matthews, S. (2001). Intimin and the host cell – is it bound to end in Tir(s)? Trends Microbiol 9, 214–218. Giron, J. A., Ho, A. S. & Schoolnik, G. K. (1991). An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254, 710–713. Giron, J. A., Ho, A. S. & Schoolnik, G. K. (1993). Characterization of fimbriae produced by enteropathogenic Escherichia coli. J Bacteriol 175, 7391–7403. ACKNOWLEDGEMENTS Giron, J. A., Torres, A. G., Freer, E. & Kaper, J. B. (2002). The flagella We thank Ilan Rosenshine for the H6 flagella antiserum and the Wellcome Trust for financial support. M. S. D. and L.-C. L. were supported by Public Health Service Awards AI37606 and AI32074 from the National Institutes of Health. of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol Microbiol 44, 361–379. Hartland, E. L., Batchelor, M., Delahay, R. M., Hale, C., Matthews, S., Dougan, G., Knutton, S., Connerton, I. & Frankel, G. (1999). Binding of intimin from enteropathogenic Escherichia coli to Tir and to host cells. Mol Microbiol 32, 151–158. REFERENCES Anantha, R. P., Stone, K. D. & Donnenberg, M. S. (2000). Effects of bfp mutations on biogenesis of functional enteropathogenic Escherichia coli type IV pili. J Bacteriol 182, 2498–2506. Badea, L., Doughty, S., Nicholls, L., Sloan, J., Robins-Browne, R. M. & Hartland, E. L. (2003). Contribution of Efa1/LifA to the adherence of enteropathogenic Escherichia coli to epithelial cells. Microb Pathog 34, 205–215. Batchelor, M., Knutton, S., Caprioli, A., Huter, V., Zanial, M., Dougan, G. & Frankel, G. (1999). Development of a universal intimin antiserum and PCR primers. J Clin Microbiol 37, 3822–3827. Bieber, D., Ramer, S. W., Wu, C. Y., Murray, W. J., Tobe, T., Fernandez, R. & Schoolnik, G. K. (1998). Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280, 2114–2118. Campellone, K. G. & Leong, J. M. (2003). Tails of two Tirs: actin Hicks, S., Frankel, G., Kaper, J. B., Dougan, G. & Phillips, A. D. (1998). Role of intimin and bundle-forming pili in enteropathogenic Escherichia coli adhesion to pediatric intestinal tissue in vitro. Infect Immun 66, 1570–1578. Kaper, J. B. (1996). Defining EPEC. Rev Microbiol Sao Paulo 27, 130–133. Kenny, B., Lai, L. C., Finlay, B. B. & Donnenberg, M. S. (1996). EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol Microbiol 20, 313–323. Kenny, B., DeVinney, R., Stein, M., Reinscheid, D. J., Frey, E. A. & Finlay, B. B. (1997). Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91, 511–520. Klapproth, J. M., Scaletsky, I. C., McNamara, B. P., Lai, L. C., Malstrom, C., James, S. P. & Donnenberg, M. S. (2000). A large toxin from pathogenic Escherichia coli strains that inhibits lymphocyte activation. Infect Immun 68, 2148–2155. pedestal formation by enteropathogenic E. coli and enterohemorrhagic E. coli O157 : H7. Curr Opin Microbiol 6, 82–90. Knutton, S. (1995). Electron microscopical methods in adhesion. Collington, G. K., Booth, I. W. & Knutton, S. (1998). Rapid Knutton, S., Lloyd, D. R. & McNeish, A. S. (1987). Adhesion of modulation of electrolyte transport in Caco-2 cell monolayers by enteropathogenic Escherichia coli (EPEC) infection. Gut 42, 200–207. enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun 55, 69–77. Cravioto, A., Gross, R. J., Scotland, S. M. & Rowe, B. (1979). Knutton, S., Baldwin, T., Williams, P. H. & McNeish, A. S. (1989). An adhesive factor found in strains of Escherichia belonging to the traditional infantile enteropathogenic serotype. Curr Microbiol 3, 95–99. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun 57, 1290–1298. Daniell, S. J., Takahashi, N., Wilson, R. & 7 other authors (2001). Knutton, S., Rosenshine, I., Pallen, M. J., Nisan, I., Neves, B. C., Bain, C., Wolff, C., Dougan, G. & Frankel, G. (1998). A novel EspA- The filamentous type III secretion translocon of enteropathogenic Escherichia coli. Cell Microbiol 3, 865–871. Donnenberg, M. S. & Kaper, J. B. (1991). Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun 59, 4310–4317. Methods Enzymol 253, 145–158. associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J 17, 2166–2176. Donnenberg, M. S., Giron, J. A., Nataro, J. P. & Kaper, J. B. (1992). Knutton, S., Shaw, R. K., Anantha, R. P., Donnenberg, M. S. & Zorgani, A. A. (1999). The type IV bundle-forming pilus of A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol Microbiol 6, 3427–3437. enteropathogenic Escherichia coli undergoes dramatic alterations in structure associated with bacterial adherence, aggregation and dispersal. Mol Microbiol 33, 499–509. Ebel, F., Podzadel, T., Rohde, M., Kresse, A. U., Kramer, S., Deibel, C., Guzman, C. A. & Chakraborty, T. (1998). Initial binding Knutton, S., Shaw, R. & Frankel, G. (2002). Interaction of of Shiga toxin-producing Escherichia coli to host cells and subsequent http://mic.sgmjournals.org enteropathogenic Escherichia coli with red blood cell monolayers. Methods Enzymol 358, 350–355. Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Sat, 17 Jun 2017 05:29:40 537 J. Cleary and others Levine, M. M., Berquist, E. J., Nalin, D. R., Waterman, D. H., Hornick, R. B., Young, C. R., Stoman, S. & Rowe, B. (1978). Escherichia coli coli type III secretion system and its direct interaction with the EspAsheath-like structure. Proc Natl Acad Sci U S A 98, 11638–11643. that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet 1 (8074), 119–122. Shaw, R. K., Daniell, S., Ebel, F., Frankel, G. & Knutton, S. (2001). Levine, M. M., Nataro, J. P., Karch, H., Baldini, M. M., Kaper, J. B., Black, R. E., Clements, M. L. & O’Brien, A. D. (1985). The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis 152, 550–559. McDaniel, T. K., Jarvis, K. G., Donnenberg, M. S. & Kaper, J. B. (1995). A genetic locus of enterocyte effacement conserved among EspA-filament-mediated protein translocation into red blood cells. Cell Microbiol 3, 213–222. Shaw, R. K., Daniell, S., Frankel, G. & Knutton, S. (2002). Entero- pathogenic Escherichia coli translocate functional Tir and form an intimin-Tir intimate attachment to red blood cell membranes. Microbiology 148, 1355–1365. Sinclair, H. B. & O’Brien, A. D. (2002). Cell-surface localized diverse enterobacterial pathogens. Proc Natl Acad Sci U S A 92, 1664–1668. nucleolin is a eukaryotic receptor for the adhesin intimin-gamma of enterohemorrhagic Escherichia coli O157 : H7. J Biol Chem 277, 2876–2885. Moon, H. W., Whipp, S. C., Argenzio, R. A., Levine, M. M. & Giannella, R. A. (1983). Attaching and effacing activities of rabbit Tobe, T. & Sasakawa, C. (2001). Role of bundle-forming pilus of and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun 41, 1340–1351. Nataro, J. P. & Kaper, J. B. (1998). Diarrheagenic Escherichia coli. enteropathogenic Escherichia coli in host cell adherence and in microcolony development. Cell Microbiol 3, 579–585. Tobe, T. & Sasakawa, C. (2002). Species-specific cell adhesion of Clin Microbiol Rev 11, 142–201. enteropathogenic Escherichia coli is mediated by type IV bundleforming pili. Cell Microbiol 4, 29–42. Nataro, J. P., Kaper, J. B., Robins-Browne, R., Prado, V., Vial, P. & Levine, M. M. (1987a). Patterns of adherence of diarrheagenic Yona-Nadler, C., Umanski, T., Aizawa, S.-I., Friedberg, D. & Rosenshine, I. (2003). Integration host factor (IHF) mediates Escherichia coli to HEp-2 cells. Pediatr Infect Dis J 6, 829–831. Nataro, J. P., Maher, K. O., Mackie, P. & Kaper, J. B. (1987b). Characterization of plasmids encoding the adherence factor of enteropathogenic Escherichia coli. Infect Immun 55, 2370–2377. repression of flagella in enteropathogenic and enterohaemorrhagic Escherichia coli. Microbiology 149, 877–884. Zhang, H. Z. & Donnenberg, M. S. (1996). DsbA is required for Nougayrede, J. P., Fernandes, P. J. & Donnenberg, M. S. (2003). stability of the type IV pilin of enteropathogenic Escherichia coli. Mol Microbiol 21, 787–797. Adhesion of enteropathogenic Escherichia coli to host cells. Cell Microbiol 5, 359–372. Zhou, X., Giron, J. A., Torres, A. G., Crawford, J. A., Negrete, E., Vogel, S. N. & Kaper, J. B. (2003). Flagellin of enteropathogenic Sekiya, K., Ohishi, M., Ogino, T., Tamano, K., Sasakawa, C. & Abe, A. (2001). Supermolecular structure of the enteropathogenic Escherichia Escherichia coli stimulates interleukin-8 production in T84 cells. Infect Immun 71, 2120–2129. 538 Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Sat, 17 Jun 2017 05:29:40 Microbiology 150