* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Redox Reactions
Enantioselective synthesis wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Elias James Corey wikipedia , lookup
Marcus theory wikipedia , lookup
Metal carbonyl wikipedia , lookup
Aldol reaction wikipedia , lookup
Stille reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Ene reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Petasis reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
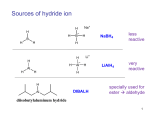
Chemistry 213 Clark College Redox Reactions: A Review Redox reactions are plentiful in organic chemistry as we learn to selectively convert one functional group into another. We will review most of the redox reactions we have learned thus far, consider some new reactions, and focus on selectively reducing or oxidizing one functional group in the presence of others. Reductions To an organic chemist, a reduction reaction is the process of adding a molecule of H2 to a molecule, typically to a pi bond. This can be achieved in three different ways: 1) Addition of H2 – catalytic hydrogenation. This method uses a metal catalyst to add H2 gas to a molecule. This works primarily on nonpolar and slightly polar molecules. 2) Addition of 2e-, 2H+. This method uses alkali metals as an electron source in conjunction with a protic solvent. 3) Addition of H-, H+ – hydride reagents. Hydride reactions are nucleophilic reactions that reduce polar compounds. They are typically followed by an aqueous or acidic work-up that provides the proton. Addition of H2 These reductions rely on transition metal catalysts to split a molecule of H2 and to provide a template for the pi bond and the H atom to react. Since they happen on the surface of the metal catalyst, the addition of H2 is a syn addition, with both H atoms adding to the same face of the pi bond. For most functional groups, a whole host of transition metals will work as catalysts. The traditional catalysts are Pt, Pd, or Ni. H2 Pt, Pd or Ni Carbon-Carbon bonds: H2 Pt, Pd or Ni H2 Lindlar's Catalyst C N H2 Ni R C N H2 Ni Carbon-Nitrogen bonds: O Carbon-Oxygen bonds: R H H2 Ni R' H2 Ni O R Redox Review Notes *Lindlar's catalyst is a "poisoned" Pd catalyst - Pd/CaCO3, Pb2+ H C N H2 R C R H NH2 OH OH R R' Page 1 of 4 Chemistry 213 Clark College Since many functional groups can be reduced with H2 and a metal catalyst, how do we get selectivity? One method would be to protect an aldehyde or ketone, perform the reduction, and then deprotect the carbonyl. However, this takes 3 synthetic steps, and is not an option for the nitrogen functional groups. To selectively reduce an alkene, in the presence of another functional group, a rhodium catalyst is employed. O O H2 Rh Addition of 2e-, 2H+ Alkali metal reductions are used for the stereochemistry of the reduction: the addition of an electron to each side of the double bond forces a “trans” configuraton of the addition of H2. Na0 NH3, -78°C Addition of H-, H+ Hydride reagents provide H- as a nucleophile, therefore they only react with polar substrates containing an electrophile. They do not react with alkenes or alkynes, so they provide additional selectivity between the functional groups. The reaction mechanism involves the nucleophilic attack of the carbonyl carbon by H-, creating an O-. An acid or aqueous work-up protonates the O- to form the alcohol. Sodium borohydride (NaBH4) is a milder reducing agent that is easier to use, but less reactive. It reacts only with the most reactive carbonyl compounds – aldehydes, ketones and some acid chlorides. O H 1) NaBH4 2) H3O+ O 1) NaBH4 2) H3O+ O 1) NaBH4 2) H3O+ Cl OH OH *Acid chlorides are the OH only acid derivative that will react with NaBH4. In order to reduce the less-reactive acids and derivatives, a stronger, more polar reducing agent is used: Lithium aluminum hydride (LiAlH4). This reagent is extremely reactive and air and moisture sensitive, and is used only when required by the starting material. O OEt O NH2 1) LiAlH4 2) H2O OH 1) LiAlH4 2) H2O NH2 Note that imines and nitriles are also reduced to amines by lithium aluminum hydride. Acid derivatives are reduced all the way to the alcohol with LiAlH4. Is there a way to stop at the aldehyde, and reduce these molecules “half-way”? The reduction still requires a hydride reagent, but one that has been modified to control stoichiometry. This reagent, DIBALH, is also used specifically with esters, which have a lower reactivity than other acid derivatives. Redox Review Notes Page 2 of 4 Chemistry 213 O OEt Clark College 1) DIBALH, -78°C 2) H2O O DIBALH = diisobutyl aluminum hydride = H Al H If we look back at the carbonyl reductions, most (except the nitrogen-containing compounds) convert the carbonyls to alcohols, so the oxygen in the molecule is retained. There are two reductions that convert carbonyls down to a –CH2– group, completely removing the oxygen. These reductions work specifically on aldehydes and ketones, and differ by being in either acidic or basic conditions, which allow for compatibility with other functional groups. O Clemmensen Reduction H O Wolff-Kishner Reduction H Zn(Hg) HCl H H N2H4 KOH A summary CN H2N H2 Ni O O Et O OH CN Redox Review Notes Et NH2 1) LiAlH4 2) H2O 1) NaBH4 2) H3O+ O O O Et O H2 Rh CN O OH OH O O Et OH Page 3 of 4 Chemistry 213 Clark College Oxidation Reactions The oxidation reactions that we will review will not be adding initial oxygens to a molecule, rather most will increase bonding to existing oxygens or adding secondary ones. We will first consider the various chromium oxidations, and then look at an aldehyde-specific reaction – the Tollens’ test. We need to remember that for an oxidation to take place, there needs to be a hydrogen on the carbon attached to the oxygen – 3° alcohols and ketones cannot be oxidized because they lack this hydrogen. Chromium Oxidations The most common, and strongest oxidizing agent is chromic acid, H2CrO4. This reagent is often generated in situ by dissolving either CrO3 or K2Cr2O7 in aqueous acidic solution. Because of the aqueous medium that the reactions take place in, chromic acid oxidations are complete – each starting material is oxidized to the highest possible oxidation state. To stop at an intermediate oxidation state, chromium can still be employed, however water must be removed. An alternate reagent, pyridinium chlorochromate (PCC) has been developed to satisfy these requirements. The table below summarizes chromium oxidations. OH PCC pyridine O O H2CrO4 H OH H2CrO4 OH H2CrO4 O or PCC/pyr Tollens’ Test The Tollens test is a reaction that is specific to aldehydes – the reaction selectively oxidizes aldehydes to carboxylic acids. This reaction is commonly used as a chemical tests for aldehydes and reducing sugars (which contain aldehyde functional groups) as the byproduct of the reaction is metallic silver which coats the reaction vessel with a mirror. O H Redox Review Notes 1) Ag+, NH3 2) H3O+ O OH Page 4 of 4