* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Roles of Arabidopsis PARC6 in Coordination of
Survey
Document related concepts
Protein (nutrient) wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Cell membrane wikipedia , lookup
Protein moonlighting wikipedia , lookup
Signal transduction wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Magnesium transporter wikipedia , lookup
Cytokinesis wikipedia , lookup
Chloroplast wikipedia , lookup
Endomembrane system wikipedia , lookup
List of types of proteins wikipedia , lookup
Proteolysis wikipedia , lookup
Transcript
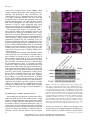
Roles of Arabidopsis PARC6 in Coordination of the Chloroplast Division Complex and Negative Regulation of FtsZ Assembly1[OPEN] Min Zhang 2, Cheng Chen 2, John E. Froehlich, Allan D. TerBush, and Katherine W. Osteryoung * Department of Plant Biology (M.Z., C.C., A.D.T., K.W.O.), Michigan State University-Department of Energy Plant Research Laboratory (J.E.F.), and Department of Biochemistry and Molecular Biology (J.E.F.), Michigan State University, East Lansing, Michigan 48824; and College of Life Sciences, Capital Normal University, Beijing 100048, China (M.Z.) ORCID IDs: 0000-0002-3716-4565 (M.Z.); 0000-0002-0028-2509 (K.W.O.). Chloroplast division is driven by the simultaneous constriction of the inner FtsZ ring (Z ring) and the outer DRP5B ring. The assembly and constriction of these rings in Arabidopsis (Arabidopsis thaliana) are coordinated partly through the inner envelope membrane protein ACCUMULATION AND REPLICATION OF CHLOROPLASTS6 (ARC6). Previously, we showed that PARC6 (PARALOG OF ARC6), also in the inner envelope membrane, negatively regulates FtsZ assembly and acts downstream of ARC6 to position the outer envelope membrane protein PLASTID DIVISION1 (PDV1), which functions together with its paralog PDV2 to recruit DYNAMIN-RELATED PROTEIN 5B (DRP5B) from a cytosolic pool to the outer envelope membrane. However, whether PARC6, like ARC6, also functions in coordination of the chloroplast division contractile complexes was unknown. Here, we report a detailed topological analysis of Arabidopsis PARC6, which shows that PARC6 has a single transmembrane domain and a topology resembling that of ARC6. The newly identified stromal region of PARC6 interacts not only with ARC3, a direct inhibitor of Z-ring assembly, but also with the Z-ring protein FtsZ2. Overexpression of PARC6 inhibits FtsZ assembly in Arabidopsis but not in a heterologous yeast system (Schizosaccharomyces pombe), suggesting that the negative regulation of FtsZ assembly by PARC6 is a consequence of its interaction with ARC3. A conserved carboxyl-terminal peptide in FtsZ2 mediates FtsZ2 interaction with both PARC6 and ARC6. Consistent with its role in the positioning of PDV1, the intermembrane space regions of PARC6 and PDV1 interact. These findings provide new insights into the functions of PARC6 and suggest that PARC6 coordinates the inner Z ring and outer DRP5B ring through interaction with FtsZ2 and PDV1 during chloroplast division. Chloroplasts evolved from an ancient cyanobacterium through endosymbiosis (Gould et al., 2008; Keeling, 2013). Like their prokaryotic relatives, chloroplasts replicate by binary fission, which is driven by a dynamic macromolecular complex located at the middle of the organelle (Falconet, 2011; Miyagishima et al., 2011; Osteryoung and Pyke, 2014). The major contractile components of the division complex include the FtsZ 1 This work was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences (grant nos. DE–FG02– 06ER15808 to K.W.O. and DE–FG02–91ER20021 to J.E.F.), the National Natural Science Foundation of China (grant no. 31470296 to M.Z.), and the U.S. National Science Foundation (grant no. 1121943 to K.W.O.). 2 These authors contributed equally to the article. * Address correspondence to [email protected]. The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Katherine W. Osteryoung ([email protected]). M.Z. and K.W.O. conceived and designed the research plans; M.Z., C.C., J.E.F., and A.D.T. performed all experiments and analyzed data; M.Z., C.C., and K.W.O wrote the article with contributions from all authors. K.W.O. supervised the research and completed the writing. [OPEN] Articles can be viewed without a subscription. www.plantphysiol.org/cgi/doi/10.1104/pp.15.01460 250 ring (Z ring), which assembles on the stromal surface of the inner envelope membrane (IEM; McAndrew et al., 2001; Vitha et al., 2001), and the DYNAMIN-RELATED PROTEIN 5B (DRP5B; also called ACCUMULATION AND REPLICATION OF CHLOROPLASTS5 [ARC5]) ring, which assembles on the cytosolic surface of the outer envelope membrane (OEM; Gao et al., 2003; Miyagishima et al., 2003; Yoshida et al., 2006). In green algae and land plants, the Z ring is composed of the tubulin-like, heteropolymer-forming proteins FtsZ1 and FtsZ2, which are both required for normal Z-ring function (Schmitz et al., 2009; TerBush and Osteryoung, 2012). DRP5B is a member of the dynamin family of membrane fission proteins, which polymerize into collar-like structures to mediate a variety of membrane fission processes in eukaryotes (Morlot and Roux, 2013). The Z ring and DRP5B ring function together to drive the simultaneous constriction of the IEM and OEM during chloroplast division. The assembly and constriction of the inner Z ring and outer DRP5B ring are coordinated across the two membranes by the activities of midplastid-localized envelope membrane proteins whose functions have been studied in Arabidopsis (Arabidopsis thaliana). ARC6 (Pyke et al., 1994) is a bitopic IEM protein of cyanobacterial origin that is conserved throughout Plant PhysiologyÒ, January 2016, Vol. 170, pp. 250–262, www.plantphysiol.org Ó 2016 American Society of Plant Biologists. All Rights Reserved. Downloaded from on June 16, 2017 - Published by www.plantphysiol.org Copyright © 2016 American Society of Plant Biologists. All rights reserved. Role of PARC6 in Chloroplast Division green-lineage chloroplasts (Koksharova and Wolk, 2002; Vitha et al., 2003; Osteryoung and Pyke, 2014). Its N-terminal region extends into the stroma, where it interacts directly and specifically with FtsZ2 (Maple et al., 2005). As FtsZ1 and FtsZ2 are soluble (McAndrew et al., 2001), this interaction probably serves both to tether the Z ring to the IEM and to promote FtsZ polymerization at the division site (Vitha et al., 2003). The C-terminal region of ARC6 protrudes into the intermembrane space (IMS) and interacts with the IMS region of the plant-specific bitopic OEM protein PLASTID DIVISION2 (PDV2). ARC6-PDV2 interaction is required for the localization of PDV2 to the midplastid (Glynn et al., 2008). PDV2 and its paralog PDV1, also in the OEM, in turn recruit DRP5B from a cytosolic pool to the OEM (Miyagishima et al., 2006), probably through direct interaction with their cytosolic regions (Holtsmark et al., 2013). Thus, interactions between FtsZ2 and ARC6 in the stroma, ARC6 and PDV2 in the IMS, and PDV2 (and PDV1) and DRP5B in the cytosol connect and coordinate the FtsZ and DRPB5B rings across the IEM and OEM. Previously, we showed that, despite the fact that an interaction between the IMS regions of ARC6 and PDV1 could not be detected, ARC6 was nevertheless required for the equatorial localization of PDV1 as well as PDV2, suggesting the existence of a factor that acted downstream of ARC6 to position PDV1 (Glynn et al., 2008). This downstream factor was subsequently shown to be the nucleus-encoded chloroplast division protein PARALOG OF ARC6 (PARC6; Glynn et al., 2009), also called CDP1 (Zhang et al., 2009) and ARC6H (Ottesen et al., 2010). parc6 mutants exhibited mislocalization of PDV1 but not PDV2, demonstrating a specific role for PARC6 in PDV1 positioning. PARC6 is restricted to vascular plants, suggesting that it arose by the duplication and divergence of ARC6 following separation of the nonvascular and vascular lineages. As suggested by its name, PARC6 shares significant sequence similarity with ARC6 and is similarly imported to the chloroplast by a cleavable N-terminal transit peptide and localized in the IEM. However, whereas ARC6 has a single transmembrane domain (TMD), PARC6 is predicted to bear two, and while a portion of its N terminus was clearly shown to reside in the stroma, its full topology has not been established (Glynn et al., 2009). Furthermore, genetic analysis suggested that, unlike ARC6, which positively regulates FtsZ assembly (Vitha et al., 2003), PARC6 functions partly as a negative regulator of FtsZ assembly. Interaction assays provided evidence that this negative regulation may be mediated by interaction of the N terminus of PARC6 with the stromal division protein ARC3 (Pyke et al., 1994; Shimada et al., 2004; Maple et al., 2007), a Z-ring positioning factor recently shown to inhibit Z-ring assembly and/or promote FtsZ filament and Z-ring destabilization (TerBush and Osteryoung, 2012; Zhang et al., 2013; Johnson et al., 2015). Although the interaction of PARC6 with FtsZ was not detected previously, the significance of this finding has remained uncertain in the absence of definitive data on PARC6 topology (Glynn et al., 2009). Here, we report a detailed topological analysis of Arabidopsis PARC6, investigate its interactions with other division factors, and assess the effect of PARC6 on chloroplast FtsZ assembly. Our findings provide evidence that the negative effect of PARC6 on Z-ring assembly results from its interaction with ARC3 and reveal a role for PARC6 in coordinating the inner Z ring and outer DRP5B ring partially analogous to the role of ARC6. RESULTS PARC6 Topology Resembles That of ARC6 As described previously (Glynn et al., 2009), PARC6 (At3g19180) is predicted to bear two TMDs: TMD1 (amino acids 357–377) and TMD2 (amino acids 574– 596; Fig. 1A, top). To analyze PARC6 topology, we carried out in vitro chloroplast import and fractionation assays on wild-type PARC6 from Arabidopsis and on a series of PARC6 variants in which TMD1, TMD2, or both were deleted (Fig. 1A). Radiolabeled precursor proteins (labeled pr in Fig. 1B) produced by in vitro translation of full-length PARC6 and the TMD deletion constructs migrated at approximately their predicted masses on SDS-PAGE gels (Fig. 1B, lane 1). When incubated with isolated chloroplasts, a significant fraction of the radiolabeled proteins migrated at a lower mass (Fig. 1B, lanes 2 and 3, labeled m), indicating protein import and transit peptide processing to yield mature protein. The smaller PARC6 and PARC6(DTMD1) import products were enriched in the membrane fraction (Fig. 1B, lane 2, arrows), while the PARC6(DTMD2) and PARC6(DTMD1/2) import products were enriched in the soluble fractions (Fig. 1B, lane 3, arrowheads). These results indicate that the association of PARC6 with the membrane fraction is mediated solely by TMD2, implying that TMD2 is the only authentic TMD in PARC6 (asterisks in Fig. 1A). Because PARC6 and ARC6 are paralogous proteins and ARC6 also has a single TMD roughly corresponding to that of PARC6 TMD2 (Vitha et al., 2003; Glynn et al., 2009; Fig. 1A, bottom), the above results suggested that Arabidopsis PARC6 is a bitopic IEM protein with a topology similar to that of ARC6 (Vitha et al., 2003): that is, with the region upstream of the authentic TMD (TMD2) oriented toward the stroma and the region downstream of this TMD situated in the IMS. To test these predictions, we conducted protease protection assays on the radiolabeled PARC6 import product (Fig. 1C, top). Following import reactions performed as described above, chloroplasts were treated either with thermolysin, which does not penetrate the OEM and therefore only degrades regions of proteins exposed to the chloroplast exterior, or with trypsin, which penetrates the OEM but not the IEM and therefore degrades regions of proteins exposed to both the chloroplast exterior and the IMS but not the stroma (Cline et al., 1984; Jackson et al., 1998). The PARC6 import product was protected from degradation by thermolysin (Fig. 1C, Plant Physiol. Vol. 170, 2016 251 Downloaded from on June 16, 2017 - Published by www.plantphysiol.org Copyright © 2016 American Society of Plant Biologists. All rights reserved. Zhang et al. top, lane 4). In contrast, trypsin treatment produced a smaller radiolabeled fragment that was associated with the membrane fraction (Fig. 1C, top, lane 6, asterisk). In control assays, a stromal protein was protected from both trypsin and thermolysin treatment and was enriched in the soluble fraction (Fig. 1C, bottom). Although proteolysis by trypsin was incomplete, the size of the trypsin-protected PARC6 fragment (Fig. 1C, top, asterisk) was consistent with the calculated molecular mass (57.3 kD) of the region of PARC6 between the end of the predicted transit peptide and the end of TMD2 (amino acids 77–596; Fig. 1A, top). These PARC6 fractionation and protease-protection patterns closely resembled those of ARC6 (Fig. 1, B, bottom, and C, middle; Vitha et al., 2003). Based on the combined data, we conclude that, similar to ARC6 (Vitha et al., 2003), Arabidopsis PARC6 is a bitopic IEM protein with its larger N-terminal region (amino acids 77–573) exposed to the stroma and its smaller C-terminal region (amino acids 597–819) exposed to the IMS. The Stromal Region of PARC6 Interacts with the Membrane Occupation and Recognition Nexus Domain of ARC3 Previously, we assessed amino acids 77 to 356 of PARC6 (PARC677-356) for interaction with the stromal chloroplast division proteins FtsZ1, FtsZ2, and ARC3 based on evidence from pea (Pisum sativum) that this region of PARC6 resides in the stroma. Only ARC3 was shown to interact (Glynn et al., 2009). Based on the topological analysis described above, we revisited these interactions by using the newly defined full-length stromal region of PARC6 (PARC677-573) in yeast twohybrid assays. As shown in Figure 2, both full-length ARC3 (lacking its predicted transit peptide) and a Figure 1. Topological analysis of Arabidopsis PARC6 at the IEM. A, Diagrams of PARC6 and ARC6 constructs used for chloroplast import assays. TP, Transit peptide; TMD, predicted TMD; *, authentic PARC6 TMD; AA, amino acids. B, In vitro [35S]PARC6, [35S]PARC6(DTMD1), [35S]PARC6(DTMD1/2), [35S]PARC6(DTMD2), and [35S]ARC6 translation products (Tr, 10% of each translation reaction loaded; lane 1) were incubated with isolated intact pea chloroplasts for 30 min (Import; lanes 2 and 3). After import, intact chloroplasts were recovered by centrifugation, lysed, and fractionated into total membrane (P; lane 2) or soluble (S; lane 3) fractions. All fractions were then analyzed by SDS-PAGE and fluorography. MM, Molecular mass markers; pr, precursor protein; m, mature import product. White arrows and arrowheads highlight the association of the import products with the membrane and soluble fractions, respectively. Lower bands in lane 1 likely resulted from initiation at internal Met codons during in vitro translation reactions (Teng et al., 2012). Where present, dark upper bands in lane 2 represent unimported precursor protein that remained associated with chloroplasts following import and recovery. C, Protease protection assays. In vitro [35S]PARC6 (top), [35S]ARC6 (middle), and [35S]SSU (Rubisco small subunit; bottom) translation products (Tr, 10% of each translation reaction loaded; lane 1) were incubated with isolated intact pea chloroplasts for 30 min (Import; lanes 2– 7). Reactions were then treated with (+) or without (2) either thermolysin or trypsin. Intact chloroplasts were recovered by centrifugation, lysed, and fractionated into total membrane (lanes 2, 4, and 6) or soluble (lanes 3, 5, and 7) fractions. All fractions were analyzed by SDS-PAGE and fluorography. *, Trypsin-protected fragments of PARC6 (top) and ARC6 (middle). 252 Plant Physiol. Vol. 170, 2016 Downloaded from on June 16, 2017 - Published by www.plantphysiol.org Copyright © 2016 American Society of Plant Biologists. All rights reserved. Role of PARC6 in Chloroplast Division The Stromal Region of PARC6 Interacts with FtsZ2 Figure 2. Yeast two-hybrid assays between PARC6 stromal regions and the stromal chloroplast division proteins FtsZ1, FtsZ2, and ARC3. Growth of Y2HGold cells was assayed in the presence (His) or absence (2His) of His to detect the activation of the HIS3 reporter. The latter medium was supplemented with 0.1 mg mL21 of the toxic drug aureobasidin A (AbA) to detect two-hybrid interactions based on activation of the AUR1-C reporter, which confers aureobasidin A resistance in the Y2HGold strain. Constructs were expressed in the pGAD-T7 (AD) or pGBK-T7 (BD) vector as indicated. Empty vectors were used as controls. Dilutions from the same starting culture are indicated at bottom. Predicted transit peptides were excluded from all constructs. Construct and strain details are described in “Materials and Methods.” C-terminal region of ARC3 bearing the membrane occupation and recognition nexus (MORN) motifs (Shimada et al., 2004), referred to as the MORN domain (amino acids 598–741; Maple et al., 2007), interacted with PARC677-573 (Fig. 2, rows 3 and 5). Deletion of the MORN domain from ARC3 abolished the interaction (Fig. 2, row 4). As with full-length ARC3 (Glynn et al., 2009), the ARC3 MORN domain alone also interacted with PARC677-356 (Fig. 2, row 17). These findings confirm that PARC6 interacts with ARC3 in yeast twohybrid assays and indicate that amino acids 77 to 356 of the newly defined PARC6 stromal region is sufficient for this interaction. These results further suggest that the PARC6-ARC3 interaction may be mediated solely by the MORN-containing C-terminal region of ARC3. We also tested whether PARC677-573 interacts with FtsZ1 and FtsZ2. As observed for PARC677-356 (Glynn et al., 2009), no interaction between FtsZ1 and PARC677-573 was detected (Fig. 2, row 1). In contrast, PARC677-573 exhibited a specific interaction with FtsZ2 (Fig. 2, row 2). Although the latter results suggested that PARC6357-573 could be responsible for the PARC6 interaction with FtsZ2, this region by itself did not support the interaction (Fig. 2, row 13), suggesting that the full-length stromal region of PARC6 or a region overlapping with both PARC677-356 and PARC6357-573 is required. To further analyze the interactions between PARC6 and the FtsZ proteins, we fused mCherry to the stromal region of PARC6 to create PARC677-573-mCherry and coexpressed it with either FtsZ1-eYFP (enhanced yellow fluorescent protein) or FtsZ2-eCFP (enhanced cyan fluorescent protein) in the fission yeast Schizosaccharomyces pombe. Recently, S. pombe has emerged as a valuable heterologous system in which to analyze the behavior of bacterial and chloroplast FtsZ filaments as well as to test the interactions with and effects of putative FtsZ assembly regulators (Srinivasan et al., 2007, 2008; TerBush and Osteryoung, 2012; Zhang et al., 2013). When expressed alone in S. pombe, PARC677-573mCherry fluorescence appeared diffusely localized in the cytosol (Fig. 3A), similar to the localization of unfused mCherry (Fig. 3B). As shown previously (TerBush and Osteryoung, 2012), FtsZ1-eYFP and FtsZ2eCFP assembled into solid cables and more elaborate filament networks, respectively (Fig. 3, C and D). In coexpression strains, PARC677-573-mCherry colocalized with FtsZ2-eCFP to a filament network similar to that formed by FtsZ2-eCFP alone (Fig. 3, D and E). We used Pearson’s correlation coefficient (PCC) to quantify the extent of overlap between the two fluorescence signals and how closely the signal intensities correlate (Bolte and Cordelières, 2006). The PARC677-573mCherry and FtsZ2-eCFP fluorescence signals had a PCC of 0.61 6 0.04 (mean 6 SE; n = 9 cells), indicating that these proteins colocalize within the filament network and that their signal intensities are directly proportional to each other (i.e. as one signal increases, so does the other). As a control, unfused mCherry localized diffusely when coexpressed with FtsZ2-eCFP (Fig. 3F), and the PCC between fluorescence signals was 0.28 6 0.05 (n = 10), indicating a low degree of colocalization. The difference in colocalization was also visually evident from the images showing the merged fluorescence signals (Fig. 3, E and F, merge). Notably, PARC677-573-mCherry did not appear to affect the formation of the FtsZ2eCFP filament network (Fig. 3, D and E), unlike ARC3, which inhibits FtsZ2 (and FtsZ1) filament assembly in S. pombe (TerBush and Osteryoung, 2012; Zhang et al., 2013). In contrast with FtsZ2-eCFP, FtsZ1-eYFP exhibited a very low degree of colocalization with PARC677-573-mCherry (Fig. 3G; PCC of 0.14 6 0.05 [n = 11]), consistent with yeast two-hybrid results (Fig. 2, row 1). Plant Physiol. Vol. 170, 2016 253 Downloaded from on June 16, 2017 - Published by www.plantphysiol.org Copyright © 2016 American Society of Plant Biologists. All rights reserved. Zhang et al. Figure 3. The stromal region of PARC6 colocalizes with FtsZ2 filaments in S. pombe. A to D, Epifluorescence and differential interference contrast (DIC) micrographs of cells expressing PARC677-573-mCherry (A), mCherry (B), FtsZ1-eYFP (C), and FtsZ2eCFP (D). E to G, Fluorescence imaging of cells coexpressing PARC677-573-mCherry and FtsZ2-eCFP (E), mCherry and FtsZ2eCFP (F), and PARC677-573-mCherry and FtsZ1-eYFP (G). eYFP and eCFP signals are falsely colored green; mCherry signals are falsely colored magenta. White areas in the merged images represent regions of signal overlap. Dotted lines indicate cell outlines. Bars = 5 mm. FtsZ2 interacts with ARC6 through a C-terminal peptide conserved among bacterial FtsZ and chloroplast FtsZ2 proteins (Vaughan et al., 2004; Maple et al., 2005). Since PARC6 is a paralog of ARC6, we tested whether the C-terminal tail of FtsZ2 mediates its interaction with PARC6 in yeast two-hybrid assays. Like ARC6 (Fig. 4A, rows 5 and 6), PARC677-573 interacted with the presumed mature form of FtsZ2 lacking its predicted transit peptide (FtsZ249-478; Olson et al., 2010; Fig. 4A, row 1) but not with a form lacking the C-terminal 18 amino acids (FtsZ249-460) that includes the conserved C-terminal peptide mentioned above (amino acids 463–478; Schmitz et al., 2009; Fig. 4A, row 2). In contrast to a previous report (Maple et al., 2005), mutation of a conserved Phe in this region (Phe-466) to Ala did not completely abolish the interaction of FtsZ2 with ARC6 (Fig. 4A, row 7). However, the FtsZ2-PARC6 interaction was abolished by the FtsZ2F466A mutation in this assay (Fig. 4A, row 3). To further evaluate these findings, we coexpressed three different versions of FtsZ2 that were C-terminally fused to the monomeric fluorescent protein mCerulean (Shaner et al., 2004; Papapetrou et al., 2009) in S. pombe and assessed the extent of their colocalization with PARC677-573-mCherry. When expressed alone, FtsZ249-478mCerulean and FtsZ2F466A-mCerulean assembled large ring-shaped structures, likely composed of loosely bundled FtsZ2 polymers (TerBush and Osteryoung, 2012; Fig. 4B, top and bottom), while FtsZ249-457-mCerulean, which lacked the C-terminal 21 amino acids of FtsZ2, assembled smaller rings and more complex networks (Fig. 4B, middle). The difference in the morphology of filaments formed by FtsZ249-478-mCerulean (Fig. 4B, top) and FtsZ2-eCFP (Fig. 3D) in S. pombe is likely due to the monomeric nature of mCerulean, as eCFP retains the intact dimer interface (Zacharias et al., 2002). In coexpression strains, PARC677-573-mCherry colocalized with FtsZ2 49-478-mCerulean with a PCC of 0.64 6 0.02 (n = 21; Fig. 4C, top row; Supplemental Fig. S1A). When coexpressed with FtsZ249-457-mCerulean, PARC677-573-mCherry localized much more diffusely (Fig. 4C, middle row) and exhibited a reduced degree of colocalization with FtsZ249-457-mCerulean (PCC of 0.53 6 0.05 [n = 23]) compared with its colocalization 254 Plant Physiol. Vol. 170, 2016 Downloaded from on June 16, 2017 - Published by www.plantphysiol.org Copyright © 2016 American Society of Plant Biologists. All rights reserved. Role of PARC6 in Chloroplast Division with FtsZ249-478-mCerulean (P = 0.056). However, a small proportion of cells still showed weak colocalization between PARC677-573-mCherry and FtsZ249-457mCerulean (Fig. 4C, middle row; Supplemental Fig. S1B, top row), suggesting that the interaction was not completely abolished. Colocalization of PARC677-573mCherry with FtsZ2F466A-mCerulean was also reduced (PCC of 0.37 6 0.05 [n = 17, P = 0.000]; Fig. 4C, bottom row; Supplemental Fig. S1C). The higher PCC value for the colocalization of PARC677-573-mCherry with FtsZ249-457-mCerulean than FtsZ2F466A-mCerulean may reflect the more extensive FtsZ2 filament network formed by the former, resulting in a higher degree of signal overlap with PARC677-573-mCherry. In control experiments, ARC668-614-mVenus, bearing the stromal region of ARC6 (Fig. 1A, bottom; Vitha et al., 2003), also had a significantly lower affinity for FtsZ249-457-mCerulean (PCC of 0.6 6 0.04 [n = 16]) and FtsZ2F466A-mCerulean (PCC of 0.51 6 0.04 [n = 21]) than FtsZ2 49-478 mCerulean (PCC of 0.8 6 0.03 [n = 14]) in S. pombe (Fig. 4E). Together with the yeast two-hybrid results, our colocalization data in S. pombe indicate that the stromal region of PARC6 interacts with FtsZ2 but not FtsZ1 and that the C-terminal tail of FtsZ2, including the conserved Phe, at least partly mediates this interaction, similar to the interaction between ARC6 and FtsZ2. Overexpression of PARC6 Inhibits FtsZ Assembly in Transgenic Plants Previously, we showed that FtsZ forms abnormally long filaments in Arabidopsis parc6-1 mutants, suggesting that PARC6 functions as a negative regulator of Z-ring assembly in wild-type plants (Glynn et al., 2009). To further investigate the role of PARC6 in chloroplast division, we generated a construct encoding a C-terminally tagged PARC6-Myc fusion protein for overexpression in Arabidopsis. To confirm functionality, we first expressed PARC6-Myc from the native PARC6 promoter Figure 4. Effect of the conserved C-terminal tail of FtsZ2 on interaction with PARC6. A, Yeast two-hybrid assays between the indicated forms of FtsZ2 and the stromal region of PARC6 (PARC677-573) or a fragment of ARC6 (ARC6154-509) shown previously to interact with FtsZ2 (Glynn et al., 2009). Growth of Y2HGold cells was assayed in the presence (+His) or absence (2His) of His to detect the activation of the HIS3 reporter. The latter medium was supplemented with 0.1 mg mL21 of the toxic drug aureobasidin A (AbA) to detect two-hybrid interactions based on the activation of the AUR1-C reporter, which confers aureobasidin A resistance in the Y2HGold strain. Constructs were expressed in the pGAD-T7 (AD) or pGBK-T7 (BD) vector as indicated. Empty vectors were used as controls. Dilutions from the same starting culture are indicated at bottom. B to E, Epifluorescence micrographs of cells expressing FtsZ249-478-mCerulean (top), FtsZ249-457-mCerulean (middle), or FtsZ2F466A-mCerulean (bottom; B); PARC677-573-mCherry and FtsZ249-478-mCerulean (top), PARC677-573-mCherry and FtsZ249-457mCerulean (middle), or PARC677-573-mCherry and FtsZ2F466A-mCerulean (bottom; C); ARC668-614-mVenus (D); or ARC668-614-mVenus and FtsZ249-478mCerulean (top), ARC668-614-mVenus and FtsZ249-457-mCerulean (middle), or ARC668-614-mVenus and FtsZ2F466A-mCerulean (bottom; E). mCherry (mCh) and mVenus (mVe) fluorescence signals are falsely colored magenta; mCerulean (mCer) fluorescence signals are falsely colored green. Dotted lines indicate cell outlines. Bars = 5 mm. Plant Physiol. Vol. 170, 2016 255 Downloaded from on June 16, 2017 - Published by www.plantphysiol.org Copyright © 2016 American Society of Plant Biologists. All rights reserved. Zhang et al. in the parc6-1 knockout mutant (SALK_100009), which exhibits enlarged chloroplasts with heterogenous morphologies and abnormal Z rings and filaments (described below; Fig. 5A; Supplemental Fig. S2A; Glynn et al., 2009). Following selection, leaf tissue from the transgenic plants was fixed and mesophyll cells were observed microscopically. The fusion protein restored both normal chloroplast size and number and the localization of FtsZ to single midplastid rings when expressed at wild-type levels (Supplemental Fig. S2, A and B), demonstrating that it retained functionality. For overexpression studies, we expressed PARC6Myc under the control of the cauliflower mosaic virus 35S promoter in wild-type Columbia-0 plants. Transgenic lines contained chloroplasts that were greatly enlarged and heterogenous in size compared with those in the wild type, and the number of chloroplasts was significantly reduced (Fig. 5A), indicating severe impairment of chloroplast division. Although these chloroplast morphology phenotypes resembled those of the parc6-1 mutant, immunoblotting with an anti-PARC6 antibody confirmed significant accumulation of the PARC6 fusion protein in these plants (Fig. 5B), indicating that overexpression of PARC6 inhibits chloroplast division. Immunofluorescence staining with antibodies against FtsZ1 and FtsZ2-1 was conducted to determine the effect of PARC6 overexpression on FtsZ assembly in plants. Midplastid-localized Z rings were detected in the wild type, whereas multiple misplaced and long FtsZ filaments were observed in parc6-1 mutants (Fig. 5A; Supplemental Fig. S2A), as reported previously (Glynn et al., 2009). In contrast, in transgenic lines with high levels of PARC6-Myc, FtsZ appeared to localize to short and disorganized filaments (Fig. 5A), suggesting a disruption of FtsZ assembly. This phenotype was not caused by altered FtsZ accumulation in these plants, because FtsZ1 and FtsZ2 protein levels were comparable to those in wild-type plants (Fig. 5B). Together, these findings reveal that overexpression of PARC6 inhibits FtsZ assembly and thus interferes with chloroplast division, consistent with parc6-1 mutant analysis suggesting that PARC6 negatively regulates assembly (Glynn et al., 2009). However, our finding that the stromal region of PARC6 does not inhibit FtsZ2 assembly in S. pombe cells (Fig. 3E) suggests that this inhibition is indirect. The IMS Regions of PARC6 and PDV1 Interact The homologous chloroplast division proteins PDV1 and PDV2 both reside in the OEM and have single TMDs and defined topologies (Miyagishima et al., 2006; Glynn et al., 2008). PDV1 has been shown to localize to the middle of deeply constricted chloroplasts and to a single spot at one pole following division, resembling the localization pattern of PARC6. Furthermore, genetic analysis has shown that PARC6 is required for the localization of PDV1 but not PDV2 to the chloroplast Figure 5. Overexpression of PARC6 disrupts Z-ring assembly in transgenic plants. A, Chloroplast phenotype images (differential interference contrast [DIC]) and immunofluorescence localization of FtsZ in mesophyll cells of the indicated genotypes. WT + 35S::PARC6-Myc, Wild-type (WT) Columbia-0 plants transformed with the 35S::PARC6-Myc construct. FtsZ1 and FtsZ2 were immunolabeled with anti-FtsZ1 and anti-FtsZ2-1 antibodies, as indicated. Green, Alexa Fluor 488-labeled goat anti-rabbit secondary antibody for anti-FtsZ1 and anti-FtsZ2-1 antibodies; magenta, chlorophyll autofluorescence. Bars are as indicated. B, Immunoblot analysis of PARC6, FtsZ1, and FtsZ2-1 in the indicated plants. Total proteins extracted from 5 mg of 9-d-old seedlings were loaded in each lane. Ponceau S-stained Rubisco large subunit (bottom) served as a loading control. Molecular mass markers (MM) are indicated on the right. division site (Glynn et al., 2009). We performed yeast two-hybrid assays to determine whether the IMS region of PDV1 (PDV1IMS) or PDV2 (PDV2IMS) would interact 256 Plant Physiol. Vol. 170, 2016 Downloaded from on June 16, 2017 - Published by www.plantphysiol.org Copyright © 2016 American Society of Plant Biologists. All rights reserved. Role of PARC6 in Chloroplast Division with the newly defined IMS region of PARC6 (PARC6IMS) described above (Fig. 1A). PARC6IMS interacted with PDV1IMS but not PDV2IMS (Fig. 6A, rows 4 and 5). To further verify the interaction with PDV1IMS, we performed in vitro pull-down assays. Maltose-binding protein (MBP)-tagged PARC6IMS expressed in Escherichia coli could be precipitated from crude E. coli extracts by glutathione-Sepharose beads coated with glutathione S-transferase (GST)-tagged PDV1IMS but not by GSTcoated or empty beads (Fig. 6B, lanes 1–3). Both PDV1 and PDV2 bear conserved Gly residues at their C termini. One of the alleles that led to the identification of PDV1 and PDV2 as chloroplast division proteins by mutant screening (pdv1-2) resulted from mutation of the PDV1 C-terminal Gly (Gly-272) to Asp, and PDV1 was mislocalized in this mutant (Miyagishima et al., 2006). A similar mutation in PDV2 greatly diminished its unique interaction with ARC6 (Glynn et al., 2008). To test whether the C-terminal Gly in PDV1 is likewise important for its interaction with PARC6, we generated PDV1IMS(G272D) constructs for use in both yeast two-hybrid and pull-down assays. PDV1IMS(G272D) failed to interact with PARC6IMS in yeast (Fig. 6A, row 7), and the same mutation significantly attenuated the binding of the PDV1 IMS region to MBP-PARC6IMS in pull-down experiments (Fig. 6B, lane 4). Together, these results strongly suggest that PARC6-dependent localization of PDV1 to the chloroplast division site is mediated by their direct interaction in the IMS and that this interaction is dependent on the C-terminal Gly in PDV1. DISCUSSION Figure 6. The IMS regions of PARC6 and PDV1 interact. A, Yeast twohybrid assay between the IMS regions of PARC6 and the PDV proteins. Growth of AH109 cells was assayed in the presence (+His) or absence (2His) of His to detect the activation of the HIS3 reporter. The latter medium was supplemented with 2.5 mM 3-amino-1,2,4-triazole (3-AT). Dilutions from the same starting culture are indicated at bottom. The PDV1IMS and PDV2IMS constructs are described by Glynn et al. (2008). The PDV1(G272D) construct is described in the text. Constructs were expressed in the pGAD-T7 (AD) or pGBK-T7 (BD) vector as indicated. B, In vitro pull-down assay of PARC6IMS and PDV1IMS. GlutathioneSepharose 4B beads were treated with buffer only (lane 1) or coated with GST (lane 2), GST-tagged PDV1IMS (lane 3), or PDV1IMS(G272D) (lane 4). The beads were then incubated with crude extracts from E. coli cells expressing MBP-PARC6IMS. Protein was eluted and analyzed by immunoblotting with anti-MBP and anti-GST antibodies. Input, Ten percent of MBP-PARC6IMS extract added to pull-down assays. A previous in vivo analysis of a PARC6 homolog in pea yielded only a partial model of PARC6 topology, which indicated that the region between the predicted transit peptide and the predicted TMD1 (Fig. 1A) was in the stromal compartment (Glynn et al., 2009). In vitro chloroplast import and protease protection assays performed here on Arabidopsis PARC6 and its TMDdeleted derivatives have now revealed that TMD2 is the only authentic TMD in PARC6 and that the previously predicted TMD1 is, in fact, part of the stromal region of PARC6. Therefore, PARC6 is a bitopic IEM protein in which the longer N-terminal region faces the stroma and the shorter C-terminal region protrudes into the IMS (Fig. 7). This topology is equivalent to that of ARC6 (Vitha et al., 2003). Both PARC6 and ARC3 function as negative regulators of FtsZ assembly, as indicated by the presence of excessively long FtsZ filaments and multiple Z rings in parc6-1 and arc3 mutants, respectively (Fig. 5; Glynn et al., 2009; Zhang et al., 2013). Consistent with these phenotypes, overexpression of both PARC6 and ARC3 in vivo had a similar inhibitory effect on FtsZ assembly and chloroplast division (Fig. 5; Zhang et al., 2009). However, PARC6 did not inhibit FtsZ2 assembly in S. pombe (Figs. 3E and 4), whereas ARC3 did (Zhang et al., 2013). Collectively, these findings indicate that the role of PARC6 as a negative regulator of FtsZ assembly in vivo is an indirect effect of its interaction with ARC3. Interestingly, the localization of FtsZ in PARC6 overexpression lines (Fig. 5) resembled that of a PARC6GFP fusion protein overexpressed in wild-type plants (Zhang et al., 2009), consistent with our data showing PARC6-FtsZ2 colocalization in S. pombe (Figs. 3E and 4). Our interaction assays based on the newly determined PARC6 topology demonstrated that the stromal region of PARC6 interacts not only with ARC3, as Plant Physiol. Vol. 170, 2016 257 Downloaded from on June 16, 2017 - Published by www.plantphysiol.org Copyright © 2016 American Society of Plant Biologists. All rights reserved. Zhang et al. Figure 7. Working model of the chloroplast division complex emphasizing the roles of PARC6 and ARC6. A, The topology of PARC6 in the IEM is equivalent to that of ARC6 (i.e. it has a single transmembrane span with its N-terminal region in the stroma and its C-terminal region in the IMS; Fig. 1). Z-ring assembly is restricted to the midplastid by the chloroplast Min system, which includes ARC3 (Glynn et al., 2007; Maple et al., 2007; Zhang et al., 2013) as well as MinD1, MinE1, and MULTIPLE CHLOROPLAST DIVISION SITE1 (not shown; Miyagishima et al., 2011; Osteryoung and Pyke, 2014). The Z ring, composed of FtsZ1/FtsZ2 heteropolymers that may assemble with mixed stoichiometries (Olson et al., 2010), is tethered to the IEM by ARC6 through direct interaction with FtsZ2 in the stroma (Maple et al., 2005; Schmitz et al., 2009). ARC6 positions PDV2 to the division site in the OEM through direct interaction of their IMS regions (Glynn et al., 2008). PARC6 functions similarly downstream of ARC6, interacting with FtsZ2 in the stroma (Figs. 2–4) and positioning PDV1 at the division site (Glynn et al., 2009) through direct interaction in the IMS (Fig. 6). PDV1 and PDV2 independently recruit DRP5B from the cytosol to the OEM, but both PDV proteins are required for full DRP5B contractile activity (Miyagishima et al., 2006), probably involving direct interactions between DRP5B and the PDV proteins and between the cytosolic regions of PDV1 and PDV2 (Holtsmark et al., 2013). Thus, PARC6 and ARC6 coordinate the Z ring and DRPB5B ring across the envelope membrane, enabling them to function together to constrict the envelope membranes. B, PARC6 recruits ARC3 to the division site, possibly during constriction. PARC6 binds to the MORN domain of ARC3 (Fig. 2; Glynn et al., 2009), allowing ARC3, an FtsZ assembly inhibitor (Zhang et al., 2013), to interact with the Z ring. This interaction may facilitate Z-ring remodeling and disassembly during constriction (Johnson et al., 2015). Other details omitted for simplicity are reviewed by Miyagishima et al. (2011) and Osteryoung and Pyke (2014). C, C terminus; N, N terminus; Z1, FtsZ1; Z2, FtsZ2. Note that proteins shown are not meant to represent stoichiometric ratios, as these have not been established. shown previously (Glynn et al., 2009), but also with FtsZ2 (Figs. 2 and 3). ARC3 prevents Z-ring misplacement in chloroplasts (Glynn et al., 2007; Maple et al., 2007), very likely by directly inhibiting the formation of Z rings distant from the midplastid division site, perhaps by destabilizing the assembly of nascent FtsZ polymers, although the mechanism remains unclear (Zhang et al., 2013; Johnson et al., 2015). Although ARC3 interacts with and inhibits the assembly of both FtsZ1 and FtsZ2 filaments, because FtsZ2 imparts stability to the Z ring, we proposed that the ARC3-FtsZ2 interaction may be particularly important in preventing Z-ring formation at nondivision sites (Zhang et al., 2013). However, both PARC6 and ARC3 localize partly to the division site (Shimada et al., 2004; Glynn et al., 2009). Our findings that the N-terminal portion of the PARC6 stromal region (PARC677-356) binds directly to the ARC3 MORN domain (Fig. 2), and that this interaction requires the MORN domain (Glynn et al., 2009), suggest that PARC6 may recruit ARC3 to the division site via this domain. In contrast, the MORN domain inhibits the interaction between ARC3 and FtsZ2 (and FtsZ1; Zhang et al., 2013). Together, these data suggest a model in which PARC6 may promote ARC3 activity in vivo by sequestering the MORN domain, thus freeing ARC3 to interact with FtsZ filaments (Fig. 7B). This hypothesis will be tested in future experiments. Although we do not yet know the significance of ARC3 localization to the division site in wild-type plants, it is possible that PARC6-ARC3-FtsZ2 interactions could promote Z-ring remodeling and disassembly during chloroplast constriction, perhaps by limiting the reassembly of FtsZ filaments as division proceeds. The interaction of FtsZ2 with PARC6 (Figs. 2 and 3E) might facilitate this activity by bringing ARC3 and FtsZ2 into close proximity (Fig. 7B). Because the PARC6 localization pattern implies that it remains associated with the newly formed poles immediately following separation of the daughter plastids (Glynn et al., 2009), PARC6, by recruiting ARC3 to the midplastid, could also (or instead) play a role in preventing the premature assembly and misplacement of Z rings at the new poles once division is complete (Osteryoung and Pyke, 2014). ARC6, which also localizes to the midplastid in the IEM (Vitha et al., 2003), has been proposed to function partly as a membrane tether for the Z ring by virtue of its interaction with FtsZ2 (Maple et al., 2005). Work on bacterial FtsZ has shown that membrane tethering is an integral part of the mechanism driving Z-ring-mediated membrane constriction (Osawa et al., 2008). However, 258 Plant Physiol. Vol. 170, 2016 Downloaded from on June 16, 2017 - Published by www.plantphysiol.org Copyright © 2016 American Society of Plant Biologists. All rights reserved. Role of PARC6 in Chloroplast Division single-particle tracking of FtsZ filaments in an arc6 mutant, while supporting such a role for ARC6, also suggested the presence of an additional membrane anchor that interacts transiently with FtsZ (Johnson et al., 2013). Our finding that FtsZ2 also interacts with PARC6 implicates PARC6 as the alternative anchor and suggests that Z-ring tethering by PARC6 could contribute to IEM constriction during chloroplast division (Fig. 7). However, in arc6 mutants, where PARC6 is presumably still present, FtsZ localizes to very small filaments and puncta and chloroplast division is drastically impaired (McAndrew et al., 2001; Vitha et al., 2003), whereas in parc6-1 mutants, where ARC6 is presumably still present, FtsZ forms multiple Z rings and spirals and chloroplasts still divide to some extent (Fig. 5; Supplemental Fig. S2; Glynn et al., 2009). These findings, along with the related ARC6 and PARC6 overexpression phenotypes (Vitha et al., 2003; Fig. 5A), indicate that ARC6 likely plays a more important role than PARC6 in Z-ring anchoring and membrane constriction. Intriguingly, the C-terminal peptide of FtsZ2, which is conserved in bacterial FtsZ, mediates FtsZ2 interaction with both PARC6 and ARC6 (Fig. 4; Maple et al., 2005). These results suggest the possibility of competitive binding of FtsZ filaments by these two proteins, which could be important in regulating Z-ring dynamics during constriction. Previously, we demonstrated that PARC6 acts genetically downstream of ARC6 to direct the midplastid localization of PDV1 (Glynn et al., 2009), but the molecular basis of this process was unclear. Analysis of PARC6 topology enabled us to identify the IMS region of PARC6 and establish that it interacts specifically with the IMS region of PDV1 but not PDV2. Similar to the specific interaction between ARC6 and PDV2 (Glynn et al., 2008), mutation of a conserved C-terminal Gly drastically attenuated this interaction (Fig. 6). Because the same mutation in pdv1-2 abolished PDV1 localization to the division site in vivo (Miyagishima et al., 2006), our results provide strong evidence that direct interaction between PARC6 and PDV1 in the IMS positions PDV1 to the midplastid during chloroplast division. These findings, together with our results showing that PDV1 can recruit DRP5B from the cytosol to the OEM in a PDV2independent manner (Miyagishima et al., 2006), possibly via direct interaction (Holtsmark et al., 2013), and that PARC6 interacts with FtsZ2 in the stroma (Figs. 2 and 3), strongly support a role for PARC6 in coordinating the inner Z ring and the outer DRP5B ring across the envelope membranes during chloroplast division in Arabidopsis (Fig. 7A). CONCLUSION Although PARC6 presumably arose in vascular plants by the duplication of ARC6 (Glynn et al., 2009) and has retained an ARC6-like topology and partially analogous role in coordination of the division machinery, PARC6 and ARC6 have evolved antagonistic functions in regulating Z-ring dynamics. ARC6 positively regulates FtsZ assembly, probably at least in part by tethering FtsZ to the IEM at the division site (Vitha et al., 2003; Maple et al., 2005), while PARC6, despite its interaction with FtsZ2, functions overall as a negative regulator of FtsZ assembly (Glynn et al., 2009), most likely as a consequence of its interaction with ARC3. Furthermore, ARC6 and PARC6 have evolved unique roles in positioning the paralogous proteins PDV2 and PDV1, respectively, at the division site. Thus, the evolution of PARC6 and PDV1 via the duplication of ARC6 and PDV2 in vascular plants (Miyagishima et al., 2006; Glynn et al., 2009; Okazaki et al., 2009; Osteryoung and Pyke, 2014) has increased the complexity of the chloroplast division machinery in this group of organisms. Future studies on the physical and functional interactions between PARC6, ARC6, and their binding partners will yield further insight into their distinct roles in the division process. MATERIALS AND METHODS Plasmid Construction for Chloroplast Import, Yeast Two-Hybrid, and Schizosaccharomyces pombe Colocalization Experiments All fragments of chloroplast division genes used for generating new constructs for chloroplast import, yeast two-hybrid, and S. pombe experiments were amplified by PCR from complementary DNA sequences. Primers are shown in Supplemental Table S1. Details of plasmid construction are described in the relevant sections below. For constructs generated using Gibson assembly reactions (Gibson et al., 2009), specified below, PCR products were used directly. Except where indicated, other PCR products were digested with the restriction enzymes shown in Supplemental Table S1 prior to insertion into the relevant vectors. All constructs were sequenced prior to use in experiments. In Vitro Translation of Precursor Protein The entire coding sequence of Arabidopsis (Arabidopsis thaliana) PARC6 was amplified with primer set MZ1151/MZ1077 (Supplemental Table S1). The resulting product was cloned into pBluescript SK+ and was subsequently mutagenized using the Gene Tailor Site-Directed Mutagenesis System (Invitrogen) using primer sets PARC6-DTMD1-F1/PARC6-DTMD1-R1 and PARC6-DTMD2F2/PARC6-DTMD2-R2 according to the manufacturer’s protocol to generate the constructs PARC6(DTMD1) and PARC6(DTMD2), respectively. PARC6(DTMD1) was used as a template with primer set PARC6-DTMD2-F2/PARC6-DTMD2-R2 to create PARC6(DTMD1/2). The SSU and ARC6 constructs used as controls have been described previously (Olsen and Keegstra, 1992; Vitha et al., 2003). Radiolabeled precursor proteins were generated by in vitro translation in the presence of [35S]L-Met (Perkin-Elmer; catalog no. NEG009T005MC) using the TNT Coupled Reticulocyte Lysate System according to the manufacturer’s protocol (Promega). After translation, the labeled proteins were diluted with an equal volume of nonradioactive 50 mM L-Met in import buffer (50 mM HEPES-KOH, pH 8, and 330 mM sorbitol). Isolation of Pea Chloroplasts Intact chloroplasts were isolated from 8- to 12-d-old pea (Pisum sativum ‘Little Marvel’, dwarf variety; Livingston Seed) seedlings and purified over a Percoll gradient as described previously (Froehlich, 2011). Intact pea chloroplasts were reisolated and resuspended in import buffer at a concentration of 1 mg chlorophyll mL21. Chloroplast Import and Protease Protection Assays Large-scale import and protease protection assays were performed as described (Froehlich, 2011). Reactions containing 150 mL of chloroplasts (1 mg Plant Physiol. Vol. 170, 2016 259 Downloaded from on June 16, 2017 - Published by www.plantphysiol.org Copyright © 2016 American Society of Plant Biologists. All rights reserved. Zhang et al. chlorophyll mL21) prepared as above, 4 mM Mg-ATP, and 10% (v/v) of the radiolabeled precursor protein in a final volume of 450 mL of import buffer were incubated for 30 min at room temperature under room light. Reactions were then divided into three 150-mL aliquots. One portion was incubated for 30 min on ice. The other portions were incubated for 30 min on ice with either thermolysin or trypsin. Thermolysin and trypsin treatments were quenched with 50 mM EDTA or trypsin inhibitor, respectively. Chloroplasts were then recovered by centrifugation through a 40% (v/v) Percoll cushion. Recovered chloroplasts were lysed by the addition of lysis buffer (25 mM HEPES, pH 8, and 4 mM MgCl2) and then fractionated into total soluble and total membrane fractions. All fractions were subsequently analyzed using SDS-PAGE. After electrophoresis, gels were subjected to fluorography and exposed to x-ray film (Eastman Kodak). platform. After extensive washing, samples were eluted from beads into 200 mL of elution buffer (20 mM Tris-HCl, pH 7.4, 200 mM NaCl, 1 mM EDTA, and 10 mM maltose). To prevent protein degradation, an EDTA-free protease inhibitor cocktail (Roche Molecular Biochemicals) was included in the elution buffer at a concentration of one tablet per 50 mL of buffer. Approximately 12.5% of the eluted material was applied in each lane for separation on 15% SDS-PAGE gels. Separated proteins were transferred to a polyvinylidene difluoride membrane (EMD Millipore) and probed with antiMBP (New England Biolabs) or anti-GST (Sigma-Aldrich) antibodies at dilutions of 1:20,000 and 1:10,000, respectively. Blots were then incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody, and immunoreactive bands were detected by chemiluminescence (Thermo Fisher Scientific). Yeast Two-Hybrid Assays Heterologous Expression of Fluorescent Fusion Proteins in S. pombe The PARC677-573 and PARC6357-573 fragments were amplified using primer sets MZ-C001/MZ-C002 and MZ-C004/MZ-C002, respectively, and inserted into the pGAD-T7 vector (Clontech) between the NdeI and BamHI sites. The ARC3MORN construct was generated by amplifying the region encompassing amino acids 598 to 741 of ARC3 using the primer set 598F/ARC3-2 and inserting the product into the pGBK-T7 vector (Clontech). The ARC3DMORN construct (previously called ARC3-MORN) is described by Glynn et al. (2009). The PARC6597-819 fragment was amplified with the primer set JG-MiaF/JG-MiaR and inserted into pGAD-T7 between the NdeI and XmaI sites to create the PARC6IMS construct. PDV1IMS(G272D) was generated by PCR-based mutagenesis of PDV1IMS (Glynn et al., 2008) using primer set JG-294/MZ1132 and cloned into pGBK-T7 between the NdeI and XhoI sites. All other yeast two-hybrid constructs were reported previously (Glynn et al., 2008, Schmitz et al., 2009). Yeast cells were grown on plates containing synthetic dropout medium either with (SD/-Trp-Leu) or without (SD/-Trp-Leu-His) His (Clontech). Assays shown in Figures 2 and 4A were performed in the yeast strain Y2HGold (Clontech). Positive interactions in this strain were detected by activation of both the HIS3 reporter, as indicated by growth in SD/-Trp-Leu-His, and the AUR1-C reporter, as indicated by growth on 0.1 mg mL21 aureobasidin A (Clontech) included in the medium. HIS3 reporter assays shown in Figure 6 were performed in the yeast stain AH109 (Clontech) in the presence of 2.5 mM 3-amino-1,2,4-triazole (Sigma-Aldrich). Cell growth and transformation were performed according to the manufacturer’s protocol (Clontech). Interactions were tested in at least three independent assays, and consistent results were obtained. Pull-Down Assays A fragment of PDV1 encoding amino acids 226 to 272 was PCR amplified from complementary DNA using primer set JG-498 and JG-499, digested with BamHI and NotI, and then inserted into pGEX-4T-2 (GE Healthcare Life Sciences) to create the construct GST-PDV1IMS. To make GST-PDV1IMS(G272D), PDV1IMS(G272D) was generated by PCR-based mutagenesis using primers JG-498 and MZ-1132 and then cloned into pGEX-4T-2. A fragment encoding amino acids 597 to 819 of PARC6 was amplified with primers BA01 and BA02, digested by EcoRI and HindIII, and then cloned into pMAL-c4x (New England Biolabs), yielding MBP-PARC6IMS. All three constructs and the empty vectors pGEX-4T-2 encoding GST and pMAL-c4x encoding MBP were transformed into BL21 (DE3) Codon Plus cells (Stratagene). Cells harboring pGEX-4T-2, GST-PDV1IMS, or GST-PDV1IMS(G272D) were cultured in normal Luria-Bertani medium (10 g of NaCl, 10 g of tryptone, and 5 g of yeast extract per L), while cells harboring MBP-PARC6IMS were grown in low-salt Luria-Bertani medium (5 g of NaCl, 10 g of tryptone, and 5 g of yeast extract) with 0.2% (w/v) Glc. Expression of all constructs was induced with 0.5 mM isopropylthio-b-galactoside and cultured at 37°C for 3 h. Cell pellets were resuspended in lysis buffer (20 mM Tris-HCl, pH 7.4, 200 mM NaCl, and 1 mM EDTA) with EDTA-free protease inhibitor cocktail (Roche Molecular Biochemicals) and then lysed by sonication. All recombinant proteins in crude cell extracts were roughly quantified by comparison with 2 mg of bovine serum albumin protein on SDS-PAGE gels stained with Coomassie Blue. For pull-down assays, the lysis buffer only or approximately 700 mL of clarified cell lysate containing 10 mg of GST, GST-PDV1IMS, or GSTPDV1IMS(G272D) was incubated at 4°C for 4 h in 2-mL microtubes with 50 mL of equilibrated glutathione-Sepharose 4B beads (GE Healthcare Life Sciences) preequilibrated with lysis buffer. The beads in each tube were then washed extensively with lysis buffer and incubated with 500 mL of cell lysate containing approximately 10 mg of MBP-PARC6IMS at 4°C for another 4 h on a rocking The expression vectors pREP41X and pREP42X were used for all experiments in S. pombe (Basi et al., 1993; Forsburg, 1993). Sequences encoding the PARC6 stromal region (PARC677-573) and mCherry (Shaner et al., 2004) were amplified using the primer sets CC-4/CC-8 and CC-6/CC-7, respectively, and cloned into pREP41X and pREP42X digested with XhoI and BamHI using the Gibson assembly method, yielding pREP41X-PARC677-573-mCherry and pREP42XPARC677-573-mCherry. To obtain pREP41X-mCherry, the mCherry fragment was PCR amplified using primer set CC-10/CC-7 and cloned into pREP41X digested with XhoI and BamHI. The pREP41X-FtsZ1-eYFP and pREP42X-FtsZ2eCFP constructs and cell growth and transformation conditions were described previously (TerBush and Osteryoung, 2012). To obtain pREP42X-FtsZ249-478mCerulean, FtsZ249-478 and mCerulean (Shaner et al., 2004; Papapetrou et al., 2009; Addgene) fragments were PCR amplified using primer sets AT264/AT299 and AT7/AT297, respectively, and cloned into BamHI-digested pREP42X by Gibson assembly. pREP42X-FtsZ249-457-mCerulean was generated similarly using primer sets AT264/AT288 and AT7/AT297, respectively. An FtsZ2F466A-mCerulean fragment was PCR amplified from the pREP42X-FtsZ249-478-mCerulean construct using primer sets AT264/AT350 and AT349/AT297 and cloned into BamHI-digested pREP42X by Gibson assembly to generate pREP42X-FtsZ2F466A-mCerulean. To make pREP41X-ARC668-614-mVenus, ARC668-614 and mVenus (A206K mutation of Venus; D.A. Moore and H.P. Erickson, personal communication; Nagai et al., 2002; Zacharias et al., 2002) were PCR amplified using primer sets AT168/AT139 and AT95/AT147, respectively, and cloned into pREP41X digested with XhoI and BamHI by Gibson assembly. S. pombe cells expressing chloroplast division fluorescent fusion proteins were imaged by differential interference contrast and epifluorescence microscopy with a Leica DMRA2 microscope equipped with an HCX PL FLUOTAR 1003 (1.30 numerical aperture) oil-immersion objective (Leica) and a CCD camera (Retiga Exi; QImaging). Two microliters of liquid cell culture was pipetted onto a polylysine-coated slide and covered with a No. 1.5 coverslip. All images were collected at room temperature. Z stacks were taken at 0.5-mm increments, and nearest neighbor deconvolution with 70% haze removal was performed with Image-Pro Plus 7.0 software (Media Cybernetics) on Z planes with in-focus fluorescence signal. Further image manipulations were performed with Fiji (ImageJ) software (http://fiji.sc/Fiji). Projections were made from Z stacks using the max intensity algorithm and were falsely colored as indicated in the figure legends. Colocalization of PARC677-573-mCherry with FtsZ1-eYFP, FtsZ2-eCFP, FtsZ249-478-mCerulean, FtsZ249-457-mCerulean, or FtsZ2F466A-mCerulean and mCherry with FtsZ2-eCFP was quantified by creating a composite image of the two coexpressed fluorescent signals from a deconvoluted Z stack, cropping the image to contain only the cell being analyzed, unmerging the two channels, using the Coloc2 plugin within Fiji to calculate an individual PCC value, and then averaging all PCC values for each coexpression strain 6 SE. Generation of Transgenic Plants Gibson assembly (Gibson et al., 2009) was used to generate constructs for expression in transgenic plants. The bacterial artificial chromosome genomic clone MVI11 (Arabidopsis Biological Resource Center) was used as a template with primers CC-27 and CC-28 (Supplemental Table S1) to produce a PARC6Myc fragment by PCR. The PARC6-Myc fragment was cloned into the pCAMBIA1300-20 vector (Zhang et al., 2013) digested with BamHI and SacI to produce 35S::PARC6-Myc. For complementation of parc6-1, an approximately 1.5-kb genomic fragment upstream of the PARC6 start codon (Zhang et al., 260 Plant Physiol. Vol. 170, 2016 Downloaded from on June 16, 2017 - Published by www.plantphysiol.org Copyright © 2016 American Society of Plant Biologists. All rights reserved. Role of PARC6 in Chloroplast Division 2009) was amplified using primers CC-31 and CC-28. The resulting PCR product and the PARC6-Myc fragment were cloned into pCAMBIA1300-20 digested with PstI and SacI to produce PARC6::PARC6-Myc. Constructs were introduced into Agrobacterium tumefaciens GV3101 by electroporation, and plants were transformed by floral dipping (Zhang et al., 2006). T1 transgenic plants were selected on plates containing 20 mg mL21 hygromycin, then transferred to soil and grown as described (Kadirjan-Kalbach et al., 2012). Protein Extraction and Immunoblot Analysis For 35S::PARC6-Myc overexpression lines, 9-d-old T2 transgenic seedlings were selected on hygromycin as above and whole seedlings were harvested directly from plates. For PARC6::PARC6-Myc complementation lines, leaf tissue was harvested from 4-week-old T1 transgenic plants. Protein samples were prepared as described previously (Kadirjan-Kalbach et al., 2012) with slight modification. Briefly, tissue was ground to powder in liquid nitrogen using an MP FastPrep-24 Tissue and Cell Homogenizer (MP Biomedicals). The powder was suspended in 43 SDS-PAGE loading buffer (40% glycerol, 240 mM TrisHCl, pH 6.8, 8% SDS, 0.04% bromophenol blue, and 5% b-mercaptoethanol) at a concentration of 0.5 mg fresh weight mL21. Proteins were separated on 10% SDS-PAGE gels and transferred to nitrocellulose membrane. After blocking, the membrane was incubated overnight with anti-PARC6 (1:500 dilution; Glynn et al., 2009), anti-FtsZ1 (1:20,000 dilution), or anti-FtsZ2-1 (1:14,000 dilution; Stokes et al., 2000) antibodies. Following washing, blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:5,000 dilution) for 1 h at room temperature. The bands were detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific). Analysis of Chloroplast Phenotypes Samples were collected from expanded rosette leaves of 3- to 4-week-old plants. Leaf tips were fixed in 3.5% glutaraldehyde for 3 h followed by heating in 0.1 M Na2EDTA (pH 9) at 50°C for an additional 1.5 h (Pyke and Leech, 1991). Leaf samples were viewed with a Leica DMI3000B inverted microscope equipped with a Leica DFC320 camera. Immunofluorescence Staining Immunofluorescence staining of leaf samples obtained from 3- to 4-week-old plants was performed essentially as described (Yoder et al., 2007; Vitha and Osteryoung, 2011). Five-micrometer-thick sections were incubated with affinity-purified anti-FtsZ1 (1:3,500 dilution) or anti-FtsZ2-1 (1:3,500 dilution) antibodies (Yoder et al., 2007). Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (Invitrogen) was used at a dilution of 1:500. Samples were observed with a Leica DMRA2 microscope as described above using Leica filters L5 (480 nm excitation/505 nm emission) and TX2 (560 nm excitation/595 nm emission) to visualize Alexa Fluor 488 and chlorophyll fluorescence, respectively. Sequence data from this article can be found in The Arabidopsis Information Resource or GenBank/EMBL databases under the following names and accession numbers: PARC6 (AT3G19180), ARC6 (AT5G42480), ARC3 (AT1G75010), FtsZ1 (AT5G55280), FtsZ2-1 (AT2G36250), PDV1 (AT5G53280), and PDV2 (AT2G16070). The mutant used in this study is parc6-1 (SALK_100009). Supplemental Data The following supplemental materials are available. Supplemental Figure S1. Additional examples of PARC677-573-mCherry coexpressed with the various forms of FtsZ2-mCerulean. Supplemental Figure S2. Complementation of parc6-1 mutants with the PARC6-Myc fusion gene. Supplemental Table S1. Primers used in this study. ACKNOWLEDGMENTS We thank Jonathan Glynn, Aaron Schmitz, and Bradley Abramson for providing yeast two-hybrid constructs, Katie Porter for generating the FtsZ2 F466A mutation construct for use in S. pombe, Larissa Osterbaan and Geoffry Davis for conducting preliminary PARC6-PDV1 interaction assays, Maren Friesen for providing a plasmid encoding mCherry, Desmond Moore and Harold P. Erickson for providing the mVenus construct, and Chen Wang for assistance with the preparation of Figure 7. Received September 15, 2015; accepted November 2, 2015; published November 2, 2015. LITERATURE CITED Basi G, Schmid E, Maundrell K (1993) TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123: 131–136 Bolte S, Cordelières FP (2006) A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224: 213–232 Cline K, Werner-Washburne M, Andrews J, Keegstra K (1984) Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol 75: 675–678 Falconet D (2011) Origin, evolution and division of plastids. In JJ Eaton-Rye, BC Tripathy, TD Sharkey, eds, Advances in Photosynthesis and Respiration, Vol 34. Springer, Dordrecht, The Netherlands, pp 35–61 Forsburg SL (1993) Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res 21: 2955–2956 Froehlich JE (2011) Studying Arabidopsis envelope protein localization and topology using thermolysin and trypsin proteases. In RP Jarvis, ed, Chloroplast Research in Arabidopsis: Methods and Protocols. Methods in Molecular Biology, Vol 774. Humana Press, New York, pp 351–367 Gao H, Kadirjan-Kalbach D, Froehlich JE, Osteryoung KW (2003) ARC5, a cytosolic dynamin-like protein from plants, is part of the chloroplast division machinery. Proc Natl Acad Sci USA 100: 4328–4333 Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA III, Smith HO (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6: 343–345 Glynn JM, Froehlich JE, Osteryoung KW (2008) Arabidopsis ARC6 coordinates the division machineries of the inner and outer chloroplast membranes through interaction with PDV2 in the intermembrane space. Plant Cell 20: 2460–2470 Glynn JM, Miyagishima SY, Yoder DW, Osteryoung KW, Vitha S (2007) Chloroplast division. Traffic 8: 451–461 Glynn JM, Yang Y, Vitha S, Schmitz AJ, Hemmes M, Miyagishima SY, Osteryoung KW (2009) PARC6, a novel chloroplast division factor, influences FtsZ assembly and is required for recruitment of PDV1 during chloroplast division in Arabidopsis. Plant J 59: 700–711 Gould SB, Waller RF, McFadden GI (2008) Plastid evolution. Annu Rev Plant Biol 59: 491–517 Holtsmark I, Lee S, Lunde KA, Auestad K, Maple-Grødem J, Møller SG (2013) Plastid division control: the PDV proteins regulate DRP5B dynamin activity. Plant Mol Biol 82: 255–266 Jackson DT, Froehlich JE, Keegstra K (1998) The hydrophilic domain of Tic110, an inner envelope membrane component of the chloroplastic protein translocation apparatus, faces the stromal compartment. J Biol Chem 273: 16583–16588 Johnson CB, Shaik R, Abdallah R, Vitha S, Holzenburg A (2015) FtsZ1/ FtsZ2 turnover in chloroplasts and the role of ARC3. Microsc Microanal 21: 313–323 Johnson CB, Tang LK, Smith AG, Ravichandran A, Luo Z, Vitha S, Holzenburg A (2013) Single particle tracking analysis of the chloroplast division protein FtsZ anchoring to the inner envelope membrane. Microsc Microanal 19: 507–512 Kadirjan-Kalbach DK, Yoder DW, Ruckle ME, Larkin RM, Osteryoung KW (2012) FtsHi1/ARC1 is an essential gene in Arabidopsis that links chloroplast biogenesis and division. Plant J 72: 856–867 Keeling PJ (2013) The number, speed, and impact of plastid endosymbioses in eukaryotic evolution. Annu Rev Plant Biol 64: 583–607 Koksharova OA, Wolk CP (2002) A novel gene that bears a DnaJ motif influences cyanobacterial cell division. J Bacteriol 184: 5524–5528 Maple J, Aldridge C, Møller SG (2005) Plastid division is mediated by combinatorial assembly of plastid division proteins. Plant J 43: 811–823 Maple J, Vojta L, Soll J, Møller SG (2007) ARC3 is a stromal Z-ring accessory protein essential for plastid division. EMBO Rep 8: 293–299 Plant Physiol. Vol. 170, 2016 261 Downloaded from on June 16, 2017 - Published by www.plantphysiol.org Copyright © 2016 American Society of Plant Biologists. All rights reserved. Zhang et al. McAndrew RS, Froehlich JE, Vitha S, Stokes KD, Osteryoung KW (2001) Colocalization of plastid division proteins in the chloroplast stromal compartment establishes a new functional relationship between FtsZ1 and FtsZ2 in higher plants. Plant Physiol 127: 1656–1666 Miyagishima SY, Froehlich JE, Osteryoung KW (2006) PDV1 and PDV2 mediate recruitment of the dynamin-related protein ARC5 to the plastid division site. Plant Cell 18: 2517–2530 Miyagishima SY, Nakanishi H, Kabeya Y (2011) Structure, regulation, and evolution of the plastid division machinery. Int Rev Cell Mol Biol 291: 115–153 Miyagishima SY, Nishida K, Mori T, Matsuzaki M, Higashiyama T, Kuroiwa H, Kuroiwa T (2003) A plant-specific dynamin-related protein forms a ring at the chloroplast division site. Plant Cell 15: 655–665 Morlot S, Roux A (2013) Mechanics of dynamin-mediated membrane fission. Annu Rev Biophys 42: 629–649 Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A (2002) A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20: 87–90 Okazaki K, Kabeya Y, Suzuki K, Mori T, Ichikawa T, Matsui M, Nakanishi H, Miyagishima SY (2009) The PLASTID DIVISION1 and 2 components of the chloroplast division machinery determine the rate of chloroplast division in land plant cell differentiation. Plant Cell 21: 1769–1780 Olsen LJ, Keegstra K (1992) The binding of precursor proteins to chloroplasts requires nucleoside triphosphates in the intermembrane space. J Biol Chem 267: 433–439 Olson BJ, Wang Q, Osteryoung KW (2010) GTP-dependent heteropolymer formation and bundling of chloroplast FtsZ1 and FtsZ2. J Biol Chem 285: 20634–20643 Osawa M, Anderson DE, Erickson HP (2008) Reconstitution of contractile FtsZ rings in liposomes. Science 320: 792–794 Osteryoung KW, Pyke KA (2014) Division and dynamic morphology of plastids. Annu Rev Plant Biol 65: 443–472 Ottesen E, Zhong R, Lamppa GK (2010) Identification of a chloroplast division mutant coding for ARC6H, an ARC6 homolog that plays a nonredundant role. Plant Sci 178: 114–122 Papapetrou EP, Tomishima MJ, Chambers SM, Mica Y, Reed E, Menon J, Tabar V, Mo Q, Studer L, Sadelain M (2009) Stoichiometric and temporal requirements of Oct4, Sox2, Klf4, and c-Myc expression for efficient human iPSC induction and differentiation. Proc Natl Acad Sci USA 106: 12759–12764 Pyke KA, Leech RM (1991) Rapid image analysis screening procedure for identifying chloroplast number mutants in mesophyll cells of Arabidopsis thaliana (L.) Heynh. Plant Physiol 96: 1193–1195 Pyke KA, Rutherford SM, Robertson EJ, Leech RM (1994) arc6, a fertile Arabidopsis mutant with only two mesophyll cell chloroplasts. Plant Physiol 106: 1169–1177 Schmitz AJ, Glynn JM, Olson BJ, Stokes KD, Osteryoung KW (2009) Arabidopsis FtsZ2-1 and FtsZ2-2 are functionally redundant, but FtsZbased plastid division is not essential for chloroplast partitioning or plant growth and development. Mol Plant 2: 1211–1222 Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22: 1567–1572 Shimada H, Koizumi M, Kuroki K, Mochizuki M, Fujimoto H, Ohta H, Masuda T, Takamiya K (2004) ARC3, a chloroplast division factor, is a chimera of prokaryotic FtsZ and part of eukaryotic phosphatidylinositol-4phosphate 5-kinase. Plant Cell Physiol 45: 960–967 Srinivasan R, Mishra M, Murata-Hori M, Balasubramanian MK (2007) Filament formation of the Escherichia coli actin-related protein, MreB, in fission yeast. Curr Biol 17: 266–272 Srinivasan R, Mishra M, Wu L, Yin Z, Balasubramanian MK (2008) The bacterial cell division protein FtsZ assembles into cytoplasmic rings in fission yeast. Genes Dev 22: 1741–1746 Stokes KD, McAndrew RS, Figueroa R, Vitha S, Osteryoung KW (2000) Chloroplast division and morphology are differentially affected by overexpression of FtsZ1 and FtsZ2 genes in Arabidopsis. Plant Physiol 124: 1668–1677 Teng YS, Chan PT, Li HM (2012) Differential age-dependent import regulation by signal peptides. PLoS Biol 10: e1001416 TerBush AD, Osteryoung KW (2012) Distinct functions of chloroplast FtsZ1 and FtsZ2 in Z-ring structure and remodeling. J Cell Biol 199: 623–637 Vaughan S, Wickstead B, Gull K, Addinall SG (2004) Molecular evolution of FtsZ protein sequences encoded within the genomes of archaea, bacteria, and eukaryota. J Mol Evol 58: 19–29 Vitha S, Froehlich JE, Koksharova O, Pyke KA, van Erp H, Osteryoung KW (2003) ARC6 is a J-domain plastid division protein and an evolutionary descendant of the cyanobacterial cell division protein Ftn2. Plant Cell 15: 1918–1933 Vitha S, McAndrew RS, Osteryoung KW (2001) FtsZ ring formation at the chloroplast division site in plants. J Cell Biol 153: 111–120 Vitha S, Osteryoung KW (2011) Immunofluorescence microscopy for localization of Arabidopsis chloroplast proteins. In RP Jarvis, ed, Chloroplast Research in Arabidopsis: Methods and Protocols. Methods in Molecular Biology, Vol 774. Humana Press, New York, pp 33–58 Yoder DW, Kadirjan-Kalbach D, Olson BJ, Miyagishima SY, Deblasio SL, Hangarter RP, Osteryoung KW (2007) Effects of mutations in Arabidopsis FtsZ1 on plastid division, FtsZ ring formation and positioning, and FtsZ filament morphology in vivo. Plant Cell Physiol 48: 775–791 Yoshida Y, Kuroiwa H, Misumi O, Nishida K, Yagisawa F, Fujiwara T, Nanamiya H, Kawamura F, Kuroiwa T (2006) Isolated chloroplast division machinery can actively constrict after stretching. Science 313: 1435–1438 Zacharias DA, Violin JD, Newton AC, Tsien RY (2002) Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296: 913–916 Zhang M, Hu Y, Jia J, Li D, Zhang R, Gao H, He Y (2009) CDP1, a novel component of chloroplast division site positioning system in Arabidopsis. Cell Res 19: 877–886 Zhang M, Schmitz AJ, Kadirjan-Kalbach DK, Terbush AD, Osteryoung KW (2013) Chloroplast division protein ARC3 regulates chloroplast FtsZ-ring assembly and positioning in Arabidopsis through interaction with FtsZ2. Plant Cell 25: 1787–1802 Zhang X, Henriques R, Lin SS, Niu QW, Chua NH (2006) Agrobacteriummediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1: 641–646 262 Plant Physiol. Vol. 170, 2016 Downloaded from on June 16, 2017 - Published by www.plantphysiol.org Copyright © 2016 American Society of Plant Biologists. All rights reserved.