* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download White matter tract alterations in fragile X
Selfish brain theory wikipedia , lookup
Neuroinformatics wikipedia , lookup
Embodied cognitive science wikipedia , lookup
Haemodynamic response wikipedia , lookup
Time perception wikipedia , lookup
Neurolinguistics wikipedia , lookup
Neuroscience and intelligence wikipedia , lookup
Neuroplasticity wikipedia , lookup
Neuropsychology wikipedia , lookup
Neurophilosophy wikipedia , lookup
Causes of transsexuality wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Neuroanatomy wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Diffusion MRI wikipedia , lookup
Brain morphometry wikipedia , lookup
Metastability in the brain wikipedia , lookup
History of neuroimaging wikipedia , lookup
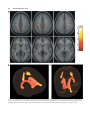
American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 118B:81 – 88 (2003) White Matter Tract Alterations in Fragile X Syndrome: Preliminary Evidence From Diffusion Tensor Imaging Naama Barnea-Goraly,1 Stephan Eliez,1 Maj Hedeus,2 Vinod Menon,1 Christopher D. White,1 Michael Moseley,2 and Allan L. Reiss1* 1 Department of Psychiatry & Behavioral Sciences, Stanford University School of Medicine, Stanford, California Department of Radiology, Stanford University School of Medicine, Stanford, California 2 Fragile X syndrome, the most common form of hereditary mental retardation, causes disruption in the development of dendrites and synapses, the targets for axonal growth in the central nervous system. This disruption could potentially affect the development, wiring, and targeting of axons. The current study utilized diffusion tensor imaging (DTI) to investigate whether white matter tract integrity and connectivity are altered in fragile X syndrome. Ten females with a diagnosis of fragile X syndrome and ten, age matched, female control subjects underwent diffusion weighted MRI scans. A whole brain analysis of fractional anisotropy (FA) values was performed using statistical parametric mapping (SPM). A follow-up, regions-of-interest analysis also was conducted. Relative to controls, females with fragile X exhibited lower FA values in white matter in fronto-striatal pathways, as well as in parietal sensory-motor tracts. This preliminary study suggests that regionally specific alterations of white matter integrity occur in females with fragile X. Aberrant white matter connectivity in these regions is consistent with the profile of cognitive and behavioral features of fragile X syndrome, Grant sponsor: NIH; Grant numbers: MH01142, HD31715, MH50047; Grant sponsor: The Packard Foundation; Grant sponsor: The Sinclair Fund; Grant sponsor: The Lynda and Scott Canel Fund for Fragile X Research. *Correspondence to: Allan L. Reiss, M.D., 401 Quarry Road, Department of Psychiatry & Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94305-5719. E-mail: [email protected] Received 17 May 2002; Accepted 4 September 2002 DOI 10.1002/ajmg.b.10035 ß 2003 Wiley-Liss, Inc. and potentially provide additional insight into the detrimental effects of suboptimal levels of FMRP in the developing brain. ß 2003 Wiley-Liss, Inc. KEY WORDS: brain; DTI; MRI INTRODUCTION Fragile X syndrome has been recognized widely as a distinct clinical syndrome only since the 1970s. However, it is now known to be the most frequent inherited form of neurodevelopmental disability in humans, with an estimated incidence of one in 3,000–4,000 [Kemper et al., 1988; Crowe and Hay, 1990; Grigsby et al., 1990; Freund, 1991; Freund et al., 1993; Mazzocco et al., 1993; Baumgardner et al., 1994]. Fragile X syndrome arises from a single gene mutation on the X chromosome, causing reduced levels of the protein, fragile X mental retardation protein (FMRP), a product of the fragile X mental retardation 1 gene (FMR1). Low or absent levels of FMRP affect physical appearance, and increase risk for psychiatric, cognitive, and behavioral disability. Physical manifestations of fragile X syndrome include a long and narrow face, large dysmorphic ears, a prominent jaw and, in males, macroorchidism [Loesch et al., 1988; Davids et al., 1990; Meryash et al., 1984]. Males are usually more affected than females and have moderate to severe mental retardation, whereas females typically have mild mental retardation or normal intellectual functioning accompanied by learning disabilities [Riddle et al., 1998]. The cognitive phenotype of fragile X syndrome includes increased risk for deficits in working memory, short term memory, visuospatial abilities, visual-motor coordination, arithmetic reasoning, and executive function [Kemper et al., 1988; Crowe and Hay, 1990; Grigsby et al., 1990; Freund, 1991; Freund et al., 1993; Mazzocco et al., 1993; Baumgardner et al., 1994]. Behaviorally, individuals with fragile X are at risk for exhibiting hyperactivity, abnormal social communication, language impairments, unusual response to sensory stimuli, gaze avoidance, anxiety, and stereotypic behavior 82 Barnea-Goraly et al. [Simon, 1990; Reiss and Freund, 1992; Lachiewicz and Dawson, 1994; Baumgardner et al., 1995; Turk and Cornish, 1998]. This recognizable cognitive, and behavioral profile associated with fragile X implies extensive involvement of FMRP in multiple brain functions and suggests that the study of individuals with this condition may provide insights into the pathophysiology of pediatric neuropsychiatric and learning disorders that share similar deficits. FMR1 mRNA expression within the brain is limited to neurons, and is highly expressed throughout the fetal and adult human brain [Devys et al., 1993; Hinds et al., 1993; Feng et al., 1997; Tamanini et al., 1997; Agulhon et al., 1999]. Within neurons, FMRP is found primarily in the perikaryon, in dendrites of all calibers, and in synapses [Devys et al., 1993; Feng et al., 1997; Weiler et al., 1997]. Early anatomical examination of the fragile X brain found no abnormalities in gross neuropathological examinations [Rudelli et al., 1985; Hinton et al., 1991]. Histologic examinations, however, detected abnormalities in the dendritic structure and density, as well as immature synapses in subjects with fragile X syndrome. These were also observed in FMR1 knockout mice [Rudelli et al., 1985; Hinton et al., 1991; Comery et al., 1997; Irwin et al., 2001]. Neuroimaging studies of fragile X syndrome indicate that the FMR1 full mutation has both a general effect on brain development and a selective effect on the growth and maintenance of specific brain regions. Specifically, studies have described increased cerebral and ventricular volumes [Reiss et al., 1991; Wisniewski et al., 1991; Schapiro et al., 1995], as well as enlarged caudate nucleus [Reiss et al., 1995a,b] and hippocampal volumes [Reiss et al., 1994; Kates et al., 1997], and decreased cerebellar vermis area [Reiss et al., 1991; Mostofsky et al., 1998]. Although FMRP is primarily expressed in the cell body and postsynaptic regions, the observed dysmorphology of dendrites and synapses in fragile X syndrome could also potentially affect the development, wiring, and targeting of axons that link affected brain regions. This, in turn, could influence white matter density and coherence between these areas. To investigate the structure of white matter tracts in fragile X syndrome we used diffusion tensor imaging (DTI), a recently developed magnetic resonance (MR) imaging technique that enables the investigation of the orientation of brain pathways in vivo. In axons, water diffusion is impeded by cell walls and myelin sheaths. As a result, water movement along the axis of an axon is much larger than water movement perpendicular to it. DTI allows visualization of this movement by fully characterizing water diffusion in three-dimensional space [Basser et al., 1994]. Since the movement of water molecules is restricted by the boundaries of the axons, visualization of that movement allows visualization of the structure and direction of axons within a DTI brain image. This study investigated white matter structure in individuals with fragile X syndrome. Based on previous neuroimaging research involving individuals with fragile X that indicated dysfunction of frontal-subcortical circuits [Hjalgrim et al., 1999], and the finding of an enlarged caudate nucleus in fragile X [Reiss et al., 1995a,b], we hypothesized that there would be white matter differences between subjects with fragile X and controls in pathways connecting the corpus striatum and the frontal lobe. METHODS Subjects Ten female subjects with a diagnosis of fragile X syndrome (age range ¼ 13.1–22.7 years; mean: 16.69 3.82) and ten, age matched, healthy females (age range ¼ 11.5–22.6 years; mean 17.05 3.82) participated in the current study. Subjects with fragile X were recruited through advertisement in newsletters. Control subjects were recruited through ads in newspapers and parent networks. All control subjects were in good health and without evidence of neurological or psychiatric disorder. Control subjects with IQ of above 130 were excluded from the study as they may not represent the average population. Females were chosen because of their higher likelihood to complete a MRI study without need of sedation [Reiss et al., 1995a,b]. After providing a complete description of the study to all participating subjects and their caretakers, written informed consent was obtained under protocols approved by the Institutional Review Board of Stanford University. The diagnosis of fragile X syndrome was confirmed by the presence of an FMR1 full mutation through DNA analysis. Standard Southern blot and polymerase chain reaction analyses were performed followed by FMR1specific probe hybridization [Oberele and Rousseau, 1991]. The CGG repeat number was calculated from the Southern blot autoradiogram images. Additionally, each participant underwent cognitive (IQ) testing using the Wechsler Intelligence Scale for Children (WISC-III) for subjects under 17 years of age, and the Wechsler Adult Intelligence Scale (WAIS-III) for subjects aged 17 and above. Image Acquisition Each participant was scanned on a 1.5 T whole body GE Signa Horizon scanner (GE medical systems, Milwaukee). A DTI sequence was based on a single-shot spin-echo echo-planar imaging (EPI) sequence with diffusion sensitizing gradients applied on either side of the 1808 refocusing pulse [Moseley et al., 1991; Basser et al., 1994]. Imaging parameters for the diffusion weighted sequence were as follows: field of view (FOV) ¼ 24 cm, matrix size 128 128, TE/TR ¼ 106/6,000 ms, 18 or 19 axial-oblique slices, slice thickness 5 mm/skip 1 mm. Seven scans in each group were zero filled to 256 256 matrix size due to database requirements at the time of acquisition. This after-the-scan manipulation did not change the resolution or the signal to noise ratio (SNR). The scan was prescribed from the top of the brain and included only the most superior part of the cerebellum. Diffusion gradient duration was d ¼ 32 ms, diffusion weighting was b ¼ 900 s/mm2. In addition, T2 weighted image were acquired by removing the diffusion sensitizing gradients. White Matter Tract Alterations in Fragile X Syndrome Diffusion was measured along six non-collinear directions: XY, XZ, YZ, XY, XZ, and YZ. This pattern was repeated four times for each slice with the sign of all diffusion gradients inverted for odd repetitions. Image Processing Raters blinded to diagnosis manually inspected the raw data images for motion artifacts and corrupted images were discarded. A maximum of two images were discarded for each direction. Eddy current effects in the diffusion weighted images (i.e., geometric distortions that vary from one diffusion direction to the next) were unwarped prior to averaging, [de Crespigny and Moseley, 1998]. Averaging of the four magnitude images efficiently removed the effect of gradient cross-terms between the diffusion sensitizing and imaging gradients [Neeman et al., 1991]. For each slice, two T2 images with no diffusion weighting (b ¼ 0 s/mm2) were acquired and averaged. In the current study, fractional anisotropy (FA) was the variable of interest. FA is an intravoxel measure that yields values between 0 (perfectly isotropic diffusion) and 1 (perfectly anisotropic diffusion) [Basser and Pierpaoli, 1996; Pierpaoli and Basser, 1996]. The degree of diffusion anisotropy in a voxel is determined by microstructural features of the tissue in that particular image voxel, including fiber diameter and density, as well as macrostructural features such as intravoxel fiber-tract coherence, and also by the degree of myelination [Pierpaoli and Basser, 1996]. The greater the diffusion anisotropy within a measured voxel—the higher the FA value. In fragile X syndrome, we expected to find reduced FA values compared to control subjects due to reduced fiber density or coherence. The FA was calculated for each voxel according to Basser and Pierpaoli [1996] to produce an FA image. The FA images were further processed using Statistic Parametric Mapping software (SPM99 software, Wellcome, UK). The T2 weighted image map was used to determine normalizing parameters subsequently applied to the FA images using SPM99. Re-sampling in the normalization process eliminates potential differences due to different matrix sizes. Normalized FA images were smoothed with a 4 mm kernel in order to increase the SNR. These smoothed images for controls and subjects with fragile X were compared using voxel-wise two-tailed t-test statistics; t-scores were normalized to Z scores to provide a statistical measure of group differences that are independent of sample size. The resultant Z score reflects the voxel-wise difference in FA between the two groups. Finally, in order to determine the presence of significant clusters of differences, the joint expected probability distribution of the height and extent of Z scores with height (Z > 2.33; P < 0.01) and extent (Z > 1.67; P < 0.05) thresholds, was used in order to correct for spatial correlation in the data [Poline et al., 1997]. Using a normalized, average SPGR image for subjects with fragile X and control subjects, a white matter cerebral mask was created and used to highlight changes in white matter tracts eliminating noise and edge effects. 83 A confirmatory analysis was subsequently conducted using regions of interest (ROIs). Spherical ROIs with a diameter of 2 mm were placed on the individual FA maps in pathways that were delineated as having lower FA values in our fragile X sample in the whole brain, voxelbased (SPM) analysis. This included the left frontalcaudate, and bilateral sensory-motor tracts. Although not reaching statistical significance in the voxel-based analysis, an ROI also was placed in the corresponding frontal-caudate tract on the right side of the brain to investigate possible laterality effects observed in fragile X subjects. Finally, ‘‘control’’ ROIs were placed bilaterally in the occipital optic radiations. A nonparametric test (Mann–Whitney U ) was used to examine group differences. A P-value of 0.01 (two-tailed) was chosen as the significance threshold. RESULTS Subjects with fragile X showed reduced FA in several brain regions compared to controls. As shown in Figure 1, one cluster was observed in left frontal-caudate white matter tracts, extending between the head of the caudate towards the prefrontal cortex. In addition, two welldefined clusters were observed along the corona radiata and centrum semiovale corresponding to sensory-motor areas bilaterally. A small cluster of increased FA in subjects with fragile X when compared with controls was seen in the posterior part of the superior temporal gyrus. Examination of group differences by ROI confirmed the significant FA differences observed in the voxelbased (SPM) analysis (Table I and Fig. 2). Significant differences in FA values between groups were seen in left frontal-caudate (U ¼ 6; P ¼ 0.009), left sensorymotor (U ¼ 5.5; P ¼ 0.0008), and in right sensory-motor tracts (U ¼ 3; P ¼ 0.0004). Although a significant cluster was not detected in the voxel-based analysis, the average FA value in the right frontal-caudate tract also was found to be significantly lower in the fragile X group (U ¼ 12.5; P ¼ 0.004). Average values generated from the ROI placed in left and right optic radiations did not differ between the two groups (left optic radiations: U ¼ 60; P ¼ 0.4; right optic radiations: U ¼ 46.5; P ¼ 0.79). IQ scores in the fragile X group ranged between 65 and 107 (mean 80.5 13.4), and in the control group between 99 and 128 (mean 115 8.6). To investigate putative brain–behavior correlations, we examined the relationship between IQ and FA within the group of subjects with fragile X with a voxel-based analysis. A few small clusters, in the superior parts of the prefrontal cortex as well as the posterior temporal lobe showed a positive correlation between IQ scores and FA values. These areas did not correspond to regions of abnormal FA in the fragile X group as compared to the healthy control group. No correlation was found between average FA values obtained in the ROI analysis and IQ scores. DISCUSSION In this study, significant differences in FA suggesting alterations in white matter density or coherence, were detected in females with fragile X syndrome. 84 Barnea-Goraly et al. Fig. 1. A: Voxels that showed significant reduction in white matter fractional anisotropy in fragile X compared to control subjects, mapped onto an average T1 weighted image of control and fragile X brains. B, C: A three-dimensional representation of the aberrant white matter tracts (shown in yellow) in relation to the caudate nucleus (shown in red), as drawn from the average image of all subjects, (B) anterior-sagittal view, (C) superior view. White Matter Tract Alterations in Fragile X Syndrome 85 TABLE I. Clusters in Which Controls had Higher Fractional Anisotropy Than Subjects With Fragile X are Shown With Their Peak Coordinates in Talairach Space and the Associated Z-Scores, Along With Their Size in Voxels FA value at the most significant voxel of difference Description of extent of cluster Right sensory-motor tracts Left caudate extending into the frontal lobe Left sensory-motor tracts Talairach coordinates of most significant voxel (x, y, z) Cluster size in voxels Z-score Fragile X group Control group 28, 20, 34 18, 23, 3 26, 38, 52 151 106 218 7.51 5.45 5.45 0.38 0.19 0.25 0.465 0.27 0.42 Specifically, the observed differences were seen in frontal-caudate circuits, as well as in sensory-motor areas bilaterally. These anisotropy differences suggest regionally specific alterations of white matter morphology in females with fragile X syndrome. Aberrant white matter connectivity in sensory-motor tracts is of potential interest to understanding deficits in sensory-processing and in sensory-integrative functions that have been observed in fragile X [Baumgardner et al., 1995; Levitas, 1996; Scharfenaker et al., 1996]. The most commonly reported manifestation of these deficits is the unusual response of individuals with fragile X syndrome to sensory stimuli, which is part of the autistic spectrum behaviors seen in individuals with this disorder [Reiss and Freund, 1990, 1992; Baumgardner et al., 1995; Levitas, 1996; Miller et al., 1999]. Early sensory-processing deficits could adversely affect important domains of development. It has been postulated that sensory-processing problems impede acquisition of higher cognitive functions, and may interfere with learning, language development, and social interactions (summarized in Scharfenaker et al., 1996). In addition, abnormal reaction to sensory stimulation have been implicated in the development and maintenance of stereotyped behavior, which is another common characteristic of individuals with fragile X syndrome [Baumgardner et al., 1995; Baranek et al., 1997]. Disruption of white matter tracts leading to brain regions involved in primary sensory-processing bilaterally, provides a possible anatomical basis for the sensory dysfunction seen in fragile X syndrome. The finding of aberrant frontal-caudate pathways in conjunction with previous observations of enlarged caudate nucleus in fragile X [Reiss et al., 1995a,b], may be associated with particular cognitive deficits, and psychiatric manifestations in fragile X syndrome. The caudate is part of several circuits that link the basal ganglia to the frontal cortex. Some of these pathways are known to play an important role in behavior and cognition [Taylor et al., 1990; Cummings, 1993; Cote and Crutcher, 2000]. Reports describing the effect of lesions within these frontal-subcortical circuits suggest that disturbances in executive function, motor programming, regulation of affect, impulse control, and flexibility in response to environmental cues can occur when these circuits are damaged [Cummings, 1993; Masterman and Cummings, 1997]. The current findings support the hypotheses put forth in previous studies of fragile X syndrome that suggest attention dysfunction, hyperactivity, executive function deficits, and impulse control problems may be related to frontal-caudate circuit abnormalities [Reiss and Denckla, 1996; Hjalgrim et al., 1999]. It is difficult to determine which circuits correspond to the areas where white matter differences were observed in our study. Future studies using fibertracking techniques will be helpful in determining the end cortical areas to which these fibers are connected and will provide more information about the potential circuits involved in fragile X. The current observation of alterations in FA in fragile X syndrome suggests that low levels of FMRP may contribute to morphological changes in white matter tracts, possibly due to an influence on neuronal growth and targeting. FMRP is present in neuronal cell bodies throughout development [Feng et al., 1997; Agulhon et al., 1999]. However, it is minimally detected in axons and not detected in glia, the components of the nervous system traditionally implicated in axonal growth and targeting [Hidalgo et al., 1995; Kalil et al., 2000; Sanes and Thomas, 2000]. Nevertheless, studies suggest an important role for the target itself in the axonal growth process. Research in animal models demonstrates that target cells from the central nervous system are important for the survival, axonal guidance, and synaptic differentiation of developing neurons [Purves, 1980; Hsiang et al., 1988]. In addition, once a connection is made, appropriate neuronal activity is essential for the correct formation of brain circuits [Katz and Shatz, 1996]. Deleterious effects of suboptimal levels of FMRP on the structure and function of synapses and dendrites, the targets for synaptic formation, may help explain the atypical white matter development in fragile X syndrome observed in the current study. An increasing body of literature investigating the role of FMRP in the brain has suggested its importance in synapse and dendrite formation. Molecular studies demonstrate high levels of FMRP in dendrites, [Devys et al., 1993; Feng et al., 1997] as well as in synapses [Feng et al., 1997; Weiler and Greenough, 1999]. Further evidence for the role of FMRP in neuronal development was recently reported by Darnell et al. [2001], who observed that FMRP–target RNAs encode proteins with roles in maintaining proper synaptic function and mediating dendritic and neuronal development. Lack of FMRP results in immature, irregularly thin, long dendritic spines in post-mortem examination of fragile X brains as well as in transgenic FMR1 knockout mice [Rudelli et al., 1985; Hinton et al., 1991; Comery et al., 1997; Irwin et al., 2001]. Braun and Segal [2000], reported the first evidence for the importance of 86 Barnea-Goraly et al. FMRP in synaptic function; electrophysiological examination of hippocampal cells from FMR1 knockout mice demonstrated that they were slower in establishing synaptic connections, and produced smaller excitatory synaptic currents than wild-type controls. Taken together, these data suggest the involvement of FMRP in dendritic spine and synaptic development and function. Although preliminary, our results suggest that disrupted synaptogenesis, whether due to a putative direct effect of FMRP on synapse maturation or to an effect on dendrite development, could disrupt correct interneuronal targeting, synaptic activity, or both. This, in Fig. 2. Boxplots show FA values from peak locations of significant clusters described in Table I (A, C, D). In right frontal-caudate tracts (B), and in a control area set in the optic radiations (E, F). White Matter Tract Alterations in Fragile X Syndrome 87 Fig. 2. (Continued) turn could result in disrupted white matter density or coherence. A clear limitation of the present study is the relatively small sample size. Further research is needed with larger numbers of subjects and, in particular, males with fragile X syndrome to confirm these preliminary results. These studies are ongoing in our laboratory. Another concern relates to previous findings of enlarged caudate and ventricular volumes in fragile X [Reiss et al., 1991, 1995a,b]. This differential morphology of ventricles and caudate nuclei might positionally shift white matter tracts in the brains of individuals with fragile X. Therefore, voxels surrounding the ventricles and the caudate nuclei in controls may contain white matter, while in subjects with fragile X the same voxels may contain gray matter, which has lower FA. These spurious FA differences might contribute to the difference in FA adjacent to the caudate nuclei, but not the FA differences seen extending from the caudate nuclei to the frontal lobes. Finally, a possible relationship between cognition and white matter anisotropy remains to be clarified. Our sample groups significantly differed in IQ scores, a finding that could potentially be associated with differences in white matter structure. However, although the females with fragile X in this study had IQ scores that spanned a broad range of abilities—from borderline IQ through the normal range, no overlap was observed between brain areas in which FA correlated with IQ and regions that showed significant between-group differences. This finding suggests that the observed white matter changes in fragile X are not directly correlated with cognitive ability. Future studies will need to examine white matter structure in individuals with fragile X syndrome and a control group with comparable IQ scores to further investigate this issue. In conclusion, this study shows preliminary evidence of white matter tract abnormalities in females with fragile X syndrome. These findings may help to provide a better understanding of the neuroanatomical and, potentially, the functional impact of decreased FMRP levels in the brain of persons with fragile X. ACKNOWLEDGMENTS The research presented in this manuscript has been supported by the following grants to Dr. Allan L. Reiss: NIH Grants MH01142, HD31715, MH50047, the Packard Foundation, the Sinclair Fund, and the Lynda and Scott Canel Fund for Fragile X Research. The author thanks Ben Krasnow, Eric Schmitt, and Leanne Tamm for their help with image processing and manuscript preparation. REFERENCES Agulhon C, Blanchet P, et al. 1999. Expression of FMR1, FXR1, and FXR2 genes in human prenatal tissues. J Neuropathol Exp Neurol 58(8):867– 880. Baranek GT, Foster LG, et al. 1997. Tactile defensiveness and stereotyped behaviors. Am J Occup Ther 51(2):91–95. Basser PJ, Pierpaoli C. 1996. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111(3):209–219. Basser PJ, Mattiello J, et al. 1994. MR diffusion tensor spectroscopy and imaging. Biophys J 66(1):259–267. 88 Barnea-Goraly et al. Baumgardner TL, Green KE, et al. 1994. A behavioral neurogenetics approach to developmental disabilities: Gene–brain-behavior associations. Curr Opin Neurol 7(2):172–178. Miller LJ, McIntosh DN, et al. 1999. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: A preliminary report. Am J Med Genet 83(4):268–279. Baumgardner TL, Reiss AL, et al. 1995. Specification of the neurobehavioral phenotype in males with fragile X syndrome. Pediatrics 95(5):744–752. Moseley ME, Wendland MF, et al. 1991. Magnetic resonance imaging of diffusion and perfusion. Top Magn Reson Imaging 3(3):50–67. Braun K, Segal M. 2000. FMRP involvement in formation of synapses among cultured hippocampal neurons. Cereb Cortex 10(10):1045–1052. Mostofsky SH, Mazzocco MM, et al. 1998. Decreased cerebellar posterior vermis size in fragile X syndrome: Correlation with neurocognitive performance. Neurology 50(1):121–130. Comery TA, Harris JB, et al. 1997. Abnormal dendritic spines in fragile X knockout mice: Maturation and pruning deficits. Proc Natl Acad Sci USA 94(10):5401–5404. Cote L, Crutcher MD. 2000. The basal ganglia. New York, NY: Elsevier. Crowe SF, Hay DA. 1990. Neuropsychological dimensions of the fragile X syndrome: Support for a non-dominant hemisphere dysfunction hypothesis. Neuropsychologia 28(1):9–16. Neeman M, Freyer JP, et al. 1991. A simple method for obtaining cross-termfree images for diffusion anisotropy studies in NMR microimaging. Magn Reson Med 21(1):138–143. Oberele I, Rousseau F. 1991. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 252:1097–1102. Cummings JL. 1993. Frontal-subcortical circuits and human behavior. Arch Neurol 50(8):873–880. Pierpaoli C, Basser PJ. 1996. Toward a quantitative assessment of diffusion anisotropy [published erratum appears in Magn Reson Med 1997 Jun;37(6):972]. Magn Reson Med 36(6):893–906. Darnell JC, Jensen KB, et al. 2001. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell 107(4):489–499. Poline JB, Worsley KJ, et al. 1997. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage 5(2):83–96. de Crespigny AJ, Moseley ME. 1998. Eddy current induced image warping in diffusion weighted EPI. Proc. ISMRM 6th Meeting, Sydney 661. Purves D. 1980. Neuronal competition. Nature 287:585–586. Devys D, Lutz Y, et al. 1993. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet 4(4):335–340. Feng Y, Gutekunst CA, et al. 1997. Fragile X mental retardation protein: Nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci 17(5):1539–1547. Reiss AL, Denckla MB. 1996. The contribution of neuroimaging to behavioral neurogenetics research: Fragile X syndrome, Turner syndrome, and neurofibromatosis-1. In: Lyon GR, XXX RJM, editors. Neuroimaging. Baltimore: Brooks. Reiss AL, Freund L. 1990. Fragile X syndrome, DSM-III-R, and autism. J Am Acad Child Adolesc Psychiatry 29(6):885–891. Freund LS, XXX RA. 1991. Cognitive profiles associated with the fra(X) syndrome in males and females. Am J Med Genet 38(4):542–547. Reiss XX, Freund XX. 1992. Behavioral phenotype of fragile X syndrome: DSM-III-R autistic behavior in male children. Am J Med Genet 43: 35–46. Freund LS, Reiss AL, et al. 1993. Psychiatric disorders associated with fragile X in the young female. Pediatrics 91(2):321–329. Reiss AL, Aylward E, et al. 1991. Neuroanatomy of fragile X syndrome: The posterior fossa. Ann Neurol 29(1):26–32. Grigsby JP, Kemper MB, et al. 1990. Neuropsychological dysfunction among affected heterozygous fragile X females. Am J Med Genet 35(1):28–35. Reiss AL, Lee J, et al. 1994. Neuroanatomy of fragile X syndrome: The temporal lobe. Neurology 44(7):1317–1324. Hidalgo A, Urban J, et al. 1995. Targeted ablation of glia disrupts axon tract formation in the Drosophila CNS. Development 121(11):3703–3712. Reiss AL, Abrams MT, et al. 1995a. Neurodevelopmental effects of the FMR-1 full mutation in humans. Nat Med 1(2):159–167. Hinds HL, Ashley CT, et al. 1993. Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome [see comments] [published erratum appears in Nat Genet 1993 Nov;5(3):312]. Nat Genet 3(1):36–43. Reiss AL, Freund LS, et al. 1995b. Contribution of the FMR1 gene mutation to human intellectual dysfunction. Nat Genet 11(3):331–334. Hinton VJ, XXX BW, Wisniewski K, Rudelli RD. 1991. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet 41(3):289– 294. Rudelli RD, XXX BW, Wisniewski K, Jenkins EC, Laura-Kamionowska M, Connell F, Wisniewski HM. 1985. Adult fragile X syndrome. Cliniconeuropathologic findings. Acta Neuropathol 67(3–4):289–295. Hjalgrim H, Jacobsen TB, et al. 1999. Frontal-subcortical hypofunction in the fragile X syndrome [letter]. Am J Med Genet 83(2):140–141. Sanes JR, Thomas MJ. 2000. The guidance of axons to their targets. Principles of neural science. In: Kandel ER, Schwartz JH, Jessell TM, editors. McGraw-Hill. 1063–1086. Hsiang J, Price SD, et al. 1988. Ultrastructural evidence for hippocampal target cell-mediated trophic effects on septal cholinergic neurons in reaggregating cell cultures. Neuroscience 26(2):417–431. Irwin SA, Patel B, et al. 2001. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: A quantitative examination. Am J Med Genet 98(2):161–167. Kalil K, Szebenyi G, et al. 2000. Common mechanisms underlying growth cone guidance and axon branching. J Neurobiol 44(2):145–158. Kates WR, Abrams MT, et al. 1997. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res 75(1):31–48. Katz LC, Shatz CJ. 1996. Synaptic activity and the construction of cortical circuits. Science 274(5290):1133–1138. Kemper MB, Hagerman RJ, et al. 1988. Cognitive profiles of boys with the fragile X syndrome. Am J Med Genet 30(1–2):191–200. Lachiewicz AM, Dawson DV. 1994. Do young boys with fragile X syndrome have macroorchidism? Pediatrics 93(6 Pt 1):992–995. Riddle JE, Cheema A, et al. 1998. Phenotypic involvement in females with the FMR1 gene mutation. Am J Ment Retard 102(6):590–601. Schapiro MB, Murphy DG, et al. 1995. Adult fragile X syndrome: Neuropsychology, brain anatomy, and metabolism. Am J Med Genet 60(6):480–493. Scharfenaker S, O’ConnorR, et al. 1996. An integrated approach to intervention. In: Hagerman R, Cronister A, editors. Fragile X syndrome. Baltimore, London: The Johns Hopkins University Press. 349–411. Simon VA. 1990. The fragile X phenotype: Cognitive, behavioral, and neurobiological profiles. Curr Opin Psychiatry 3:581–586. Tamanini F, Willemsen R, et al. 1997. Differential expression of FMR1, FXR1, and FXR2 proteins in human brain and testis. Hum Mol Genet 6(8):1315–1322. Taylor AE, Saint-Cyr JA, et al. 1990. Subcognitive processing in the frontocaudate complex loop: The role of the striatum. Alzheimer Dis Assoc Disord 4(3):150–160. Review 4(3):150–160. Turk J, Cornish K. 1998. Face recognition and emotion perception in boys with fragile-X syndrome. J Intellect Disabil Res 42(Pt 6):490–499. Levitas A. 1996. Neuropsychiatric aspects of fragile X syndrome. Semin Clin Neuropsychiatry 1(2):154–167. Weiler IJ, Greenough WT. 1999. Synaptic synthesis of the fragile X protein: Possible involvement in synapse maturation and elimination. Am J Med Genet 83(4):248–252. Masterman DL, Cummings JL. 1997. Frontal-subcortical circuits: The anatomic basis of executive, social, and motivated behaviors. J Psychopharmacol 11(2):107–114. Weiler IJ, Irwin SA, et al. 1997. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci USA 94(10):5395–5400. Mazzocco MM, Pennington BF, et al. 1993. The neurocognitive phenotype of female carriers of fragile X: Additional evidence for specificity. J Dev Behav Pediatr 14(5):328–335. Wisniewski KE, Segan SM, et al. 1991. The Fra(X) syndrome: Neurological, electrophysiological, and neuropathological abnormalities. Am J Med Genet 38(2–3):476–480.