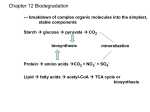
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The cell biology of lignification in higher plants
Survey
Document related concepts
Cytoplasmic streaming wikipedia , lookup
Signal transduction wikipedia , lookup
Tissue engineering wikipedia , lookup
Cell encapsulation wikipedia , lookup
Endomembrane system wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell growth wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Programmed cell death wikipedia , lookup
Cellular differentiation wikipedia , lookup
Transcript
Annals of Botany 115: 1053–1074, 2015 doi:10.1093/aob/mcv046, available online at www.aob.oxfordjournals.org REVIEW The cell biology of lignification in higher plants Jaime Barrosy, Henrik Serk1, Irene Granlundz and Edouard Pesquet* 1 Umeå Plant Science Centre (UPSC), Department of Plant Physiology, Umeå University, 901 87 Umeå, Sweden * For correspondence. E-mail [email protected] † Present address: Department of Biological Sciences, University of North Texas, 1155 Union Circle #305220, Denton, TX 76203, USA. ‡ Present address: ProTest Diagnostics, c/o Umeå Biotech Incubator AB, Tvistevägen 48c, 907 19 Umeå, Sweden. Received: 5 January 2015 Returned for revision: 23 February 2015 Accepted: 10 March 2015 Published electronically: 15 April 2015 Background Lignin is a polyphenolic polymer that strengthens and waterproofs the cell wall of specialized plant cell types. Lignification is part of the normal differentiation programme and functioning of specific cell types, but can also be triggered as a response to various biotic and abiotic stresses in cells that would not otherwise be lignifying. Scope Cell wall lignification exhibits specific characteristics depending on the cell type being considered. These characteristics include the timing of lignification during cell differentiation, the palette of associated enzymes and substrates, the sub-cellular deposition sites, the monomeric composition and the cellular autonomy for lignin monomer production. This review provides an overview of the current understanding of lignin biosynthesis and polymerization at the cell biology level. Conclusions The lignification process ranges from full autonomy to complete co-operation depending on the cell type. The different roles of lignin for the function of each specific plant cell type are clearly illustrated by the multiple phenotypic defects exhibited by knock-out mutants in lignin synthesis, which may explain why no general mechanism for lignification has yet been defined. The range of phenotypic effects observed include altered xylem sap transport, loss of mechanical support, reduced seed protection and dispersion, and/or increased pest and disease susceptibility. Key words: Lignin, lignification, non-cell autonomous processes, plant cell wall, laccases, peroxidases, monolignols, Arabidopsis thaliana. INTRODUCTION Lignin (Latin lignum ‘wood’) is a polyphenolic polymer deposited directly in the cell wall of specialized cells. It is not only restricted to plant woody tissues but represents an integral feature ensuring the proper cellular function of many other cell types in different tissues/organs of the plant. The appearance of lignin during plant evolution coincided with the emergence of the vascular land plants, or tracheophytes, in the Devonian (Weng and Chapple, 2010). Mechanically weaker than cellulose, lignin nevertheless adds a significant reinforcement to any cell wall, providing an additional tensile strength of 25–75 MPa and a Young’s modulus of 25–37 GPa (Gibson, 2012). Despite the fact that lignin is the second most abundant terrestrial biopolymer after cellulose (Boerjan et al., 2003), our understanding of lignin formation remains fragmentary. In contrast to cellulose, which presents a defined biochemical structure independently of the type of plant cell, lignin formation is cell specific and exhibits both distinct sub-cellular localization and monomeric composition: a general lignification mechanism cannot thus be drawn for all lignified cell types and may explain why lignification is still only partly understood. Our current biochemical understanding is that lignin forms in the spaces between the cellulose microfibrils by the oxidative coupling of free lignin monomers secreted directly into the plant cell wall (Boerjan et al., 2003). The canonical lignin monomers, called monolignols, are the non-methoxylated p-coumaryl alcohol, the monomethoxylated coniferyl alcohol and the dimethoxylated sinapyl alcohol which respectively form H- (hydroxyphenyl), G- (guaicyl) and S- (syringyl) units in the lignin polymer. Once these monomers are activated in the cell wall by phenoloxidases, they can displace the radical charge through their conjugated unsaturation, leading to various mesomeric resonance forms. The lignin polymer then forms by the end-wise addition of new activated monomers to its growing ends and branches (Davin and Lewis, 1992; Boerjan et al., 2003), and the different linkages between subunits (ether and carbon–carbon bonds between the aliphatic propene and/or the aromatic moieties) depend on the mesomeric form coupled. Our current lack of understanding of the biological processes behind lignin synthesis is due to the unknown mechanisms enabling the formation of distinct lignin polymers in specific cells – such as between wood fibres and vessels which are neighbours but show specific lignin accumulation and composition. Moreover, the fact that lignin cannot be removed once deposited suggests that plants require specific regulatory mechanisms to control lignin polymer biosynthesis and its subcellular localization at specific stages during the differentiation of plant cells. This review focuses on the cell biology of lignin, presenting the current knowledge of the different mechanisms controlling cell wall lignification in specific cell types. C The Author 2015. Published by Oxford University Press on behalf of the Annals of Botany Company. V All rights reserved. For Permissions, please email: [email protected] Barros et al. — The cell biology of lignification in higher plants 1054 LIGNIFIED CELL TYPES IN PLANTS Cell wall lignification occurs during the differentiation of distinct cell types, but also in response to specific environmental changes. The appropriate timing and localization of lignin deposition in each distinct cell type is essential for the proper function and adaptation of plants to their environment. Figure 1 represents a schematic view of the developmental and stressinduced lignin formation in the different cell types in intact Arabidopsis thaliana plants. The cell types accumulating lignin during their differentiation include the following. sap-conducting cylinders (Fig. 1) and they are formed by undergoing cell suicide to remove their cell content (Fukuda, 1997) and reinforcing their side walls with lateral lignified secondary cell walls mainly composed of G-units (Terashima and Fukushima, 1989; Higuchi, 1990). Genetic or pharmacological reduction of TE lignification in whole plants results in collapsed TEs due to the inability of the cell to withstand the negative pressure associated with the rising of the sap (Smart and Amrhein, 1985; Turner and Somerville, 1997; Jones et al., 2001; Thévenin et al., 2011). Tracheary elements (TEs) Sclerenchyma cells These specialized cells are an important component of the xylem: the vascular tissue responsible for the hydro-mineral sap distribution and the mechanical resistance of plants to gravity (Tyree and Zimmermann, 2002). TEs act as the plant These secondary cell wall-forming cells include fibres and sclereids, found in many different plant tissues such as xylem, phloem, epidermis and cortex in grasses and cereals, and in fruit fleshy tissues (i.e. stone cells in pear fruit; Tao et al., 2009). Development Stress Replum Wounding Pathogen attack Drought Temperature Valves Seed dispersion Nutrient availability CO2 Ozone UV radiation SILIQUE Seed coat Seed protection Replum, valves and seed coat LEAF Sclerenchyma: mechanical support Mesophyll STEM TEs: mechanical support and nutrient transport Xylem fibres and TEs Parenchyma Casparian strip ROOT Unlignified cell wall Lignified primary cell wall Lignified secondary cell wall Endodermis Endodermis: transport barrier FIG. 1. Lignified cell types in higher plants. The role of lignin: as a transport barrier; in water and nutrient transport; for mechanical support; for seed protection and dispersion; and as a response to biotic and abiotic factors. Barros et al. — The cell biology of lignification in higher plants The function of these cells is to strengthen the central axis of plant organs mechanically against gravity, mechanical disturbances and physical damage (Fig. 1). The sclerenchyma fibres and sclereids have lignified secondary cell walls mainly composed of S-units (Higuchi, 1990). Genetic modification of xylem fibre lignification by loss-of-function mutation or gene silencing results in plants unable to withstand gravity (Jones et al., 2001; Smith et al., 2013). Endodermal cells This cell type constitutes the root endodermis which delimits the root cortex from its vascular system. Endodermal cells selectively allow passage of water and solutes to the root vascular system by forming an apoplastic barrier against the extracellular diffusion of substances (Steudle, 2000; White and Broadley, 2001; Geldner, 2013): the Casparian strip. This longitudinal orientated strip, composed of lignin-like polymer combined with suberin, tightly links the plasma membranes and the apoplastic space between adjacent endodermal cells (Fig. 1). The Casparian strip lignin-like polymer is composed of a mixture of G- and S-units in monocolyledonous species, whereas dicotyledon plants exhibit more H- and G-units than S-units (Zeier and Schreiber, 1997; Zeier et al., 1999; Naseer et al., 2012). Genetic or pharmacological modification of Casparian strip lignification in whole plants leads to a loss of the apoplastic barrier function of the endodermis (Geldner, 2013). Seed coat cells During seed development, the ovule integuments differentiate into several cell layers composed of different specialized cells which will form the protective seed coat or testa (Bewley, 1997; Debeaujon et al., 2007) (Fig. 1). Seed coat cells develop heavily lignified secondary cell walls to reinforce the outer surface of the seed mechanically and to make it impermeable to liquids and gasses (Kelly et al., 1992; Liang et al., 2006; Chen et al., 2012, 2013). Seed coat lignins are different between angiosperm species (orchids and cactaceaes) and are composed of a mixture of classic G- and S-units and/or non-canonical C- (caffeyl) units which derive from an unusual monomer, caffeyl alcohol (para- and meta-hydroxylated but not methoxylated) (Tobimatsu et al., 2013). The importance of seed coat lignin for the proper function of the seed is clearly illustrated by the arabidopsis transparent testa 10 mutant, which accumulates less lignin in seeds and shows reduced germination rates after vernalization (Liang et al., 2006). However, the physicochemical properties and potential effects of lignin on embryogenesis, seed fitness, dormancy and germination still remain unknown. Siliques cells In A. thaliana and related species, the external envelope of fruits consists of three main tissues: the valves or seedpod walls; the replum or central ridge located between the valves; and the valve margins which separate the valves from the replum to disperse the seeds (Fig. 1). The release of seeds from 1055 their pods is enabled by a thin band of cells which form the dehiscence zone located between the replum and the valves. Cell wall lignification occurs specifically in valve margin cells adjacent to the dehiscence zone as well as in an internal valve cell layer (Fig. 1). During the shattering of siliques, the middle lamella between the dehiscence zone cells breaks and the separation of the cells allows the valve to separate from the replum to release the seeds. The lignin polymer composition of silique valve cells has not been reported to date. Lignin biosynthesis can also be triggered in responses to various biotic and abiotic stresses such as during wounding (Delessert et al., 2004; Kim et al., 2006), pathogen infection (Moerschbacher et al., 1990; Martı́n et al., 2007), drought (Fan et al., 2006; Yoshimura et al., 2008), UV radiation (Rozema et al., 1997; Hilal et al., 2004), low temperature (Hausman et al., 2000; El Kayal et al., 2006), reduced nutrient availability (Blodgett et al., 2005; Tahara et al., 2005) and CO2 or ozone exposure (Davey et al., 2004; Cabané et al., 2004) (Fig. 1). These stress-induced lignins generally impregnate the primary cell wall of cells that are normally not lignified (i.e. leaf epidermal or stem pith parenchyma cells) and exhibit lower amounts of S-units and higher amounts of G- and H-units as well as more condensed C–C linkages between units (Lange et al., 1995; Stange et al., 2001; Hawkins and Boudet 2003; Cabané et al., 2004). CELL BIOLOGY OF LIGNIN MONOMER SYNTHESIS Lignin deposition depends on the cell type, the developmental stage and the species. This spatial distribution is characterized by differences in time, amount, size and monomeric composition of the lignin polymer (Terashima et al., 2012). For example, TE lignin in gymnosperm wood is typically composed of G-units with a minor contribution of H-units, while in angiosperm wood, TE secondary walls contain mainly G-units and sclerenchyma fibres have G- and S-units. The overall H/G/S proportions in plant TE-rich tissues vary from 0–5/95–100/0 in gymnosperms, to 0–8/25–50/46–75 in dicot angiosperms and 5–33/33–80/20–54 in monocots/grasses (Ek et al., 2009). The pathway synthesizing these different lignin monomers combines the reduction of the c-carbon terminal function from a carboxylic acid to an alcohol and the meta and para substitution of the aromatic ring with hydroxyl and methoxyl groups. Lignin monomer precursor(s) derive from the aromatic amino acid phenylalanine, synthesized in the plastid, which is converted into 4-hydroxyphenylpropene alcohols. Monocotyledon plants also possess the capacity to use tyrosine as an additional precursor (Fig. 2). The enzymatic steps leading to the synthesis of these lignin monomers have been extensively reviewed (Boerjan et al., 2003; Bonawitz and Chapple, 2010; Vanholme et al., 2010), although new biosynthetic steps within the pathway are still being discovered. The caffeoyl shikimate esterase (CSE) (Vanholme et al., 2013), which catalyses the conversion of caffeoyl shikimic/quinic acid into caffeic acid, has been identified, as has the monocotyledon-specific p-coumaroyl-CoA:monolignol transferase (PMT) (Petrik et al., 2013) which enables the formation of c-ester-bound monolignols acetylated with p-coumarate. In addition, the mobilization and Barros et al. — The cell biology of lignification in higher plants 1056 COMT CSE PAL phenylalanine C4H/ REF3 4CL 4CL HCT ferulic acid caffeic acid tricin tyrosine TAL C3H/ REF8 feruloyl hexose CAldh/ REF1 CCoAOMT HCT feruloyl malate 4CL SGT cinnamic acid p-coumaric acid p-coumaroyl CoA CCR CCR1 p-coumaroyl shikimic / quinic aicd caffeoyl shikimic /quinic aicd caffeoyl CoA CCR CCR1 feruloyl CoA sinapic acid sinapioyl glucose CAldh/ REF1 CCR CCR1 F5H/ FAH1 COMT PMT SMT COMT cinnamoyl hexose p-coumaraldehyde caffeyl aldehyde CAD CAD coniferaldehyde CAD CAD F5H/ FAH1 COMT cinnamoyl malate p-coumaroyl alcohol caffeyl alcohol 5-hydroxyconiferaldehyde coniferyl alcohol UGT BGLU sinapaldehyde CAD sinapoyl malate COMT 5-hydroxyconiferyl alcohol sinapyl alcohol UGT BGLU Cytoplasm p-coumaroyl p-coumarate R=R’=H coniferyl p-coumarate R=H, R’=OCH3 sinapyl p-coumarate R=R’=OCH3 Lignin monomers coniferin syringin Plasma membrane BGLU Secondary cell wall H units H - H/G/S units C units G units BGLU S units Primary cell wall FIG. 2. General phenylpropanoid pathway showing lignin biosynthesis gene mutations in Arabidopsis thaliana (REF3, REF8, CCR1, REF1 and FAH1) and respective side pathway reactions (coloured boxes) affected by the mutational change. Dotted boxes represent lignin monomers that are incorporated in the lignin polymer. PAL, phenylalanine ammonia-lyase; TAL, tyrosine ammonia-lyase; C4H, cinnamate 4-hydroxylase; 4CL, 4-coumarate:CoA ligase; C3H, p-coumarate 3-hydroxylase; HCT, p-hydroxycinnamoyl-CoA:quinate/shikimate p-hydroxycinnamoyltransferase; CSE, caffeoyl shikimate esterase; CCoAOMT, caffeoyl-CoA O-methyltransferase; CCR, cinnamoyl-CoA reductase; CAD, cinnamyl alcohol dehydrogenase; COMT, caffeic acid O-methyltransferase; F5H, ferulate 5-hydroxylase; PMT, p-coumaroyl-CoA:monolignol transferase; CAldh, coniferaldehyde dehydrogenase; UGT, UDP-glucosyltransferase; BGLU, b-glucosidase; SGT, sinapic acid:UDPglucosyl sinapoyltransferase; SMT, sinapoylglucose:malate sinapoyltransferase. storage of monolignols is suggested to be regulated through glycosylation/deglycosylation involving the enzymes UDPglucosyltransferase (UGT) and b-glucosidase (BGLU) (Liu, 2012). An overall scheme including these newly identified genes/proteins is presented in Fig. 2. The phenylpropanoid pathway forms a crossroad whose branches lead to the synthesis of hormones, flavonoids, suberins and many different phenolic compounds such as (neo)lignans which derive from the same precursors as lignin (Vogt, 2010; Weng and Chapple, 2010). This crossroad position is clearly observed when key genes/enzymes of the pathway are genetically altered, leading to disturbances in both lignin and phenolic compound accumulation. The disturbances include an increase of cinnamoyl-malate in the At-REF3 mutant (loss of function Barros et al. — The cell biology of lignification in higher plants in cinnamate 4-hydroxylase, C4H; Fig. 2), an increase of feruloyl-malate in the At-CCR1 mutant (loss of function in cinnamoyl-CoA reductase, CCR; Fig. 2) or a reduction of sinapoyl-malate in the At-REF1 mutant (loss of function in coniferaldehyde dehydrogenase, CAldh; Fig. 2) as well as in the At-REF8 mutant (loss of function in p-coumaroyl shikimate 3-hydroxylase, C3H; Fig. 2) and the At-FAH1 mutant (loss of function in ferulate 5-hydroxylase, F5H; Fig. 2) (Nair et al., 2002; Mir Derikvand et al., 2008; Bonawitz and Chapple, 2010). Tissue/cell-specific expression of lignin monomer biosynthetic genes During plant development, lignin monomer biosynthetic genes are expressed in all lignifying tissues (endodermis and xylem) as well as in non-lignifying tissues such as the phloem parenchyma and the epidermis. In lignifying tissues such as xylem, lignin monomer biosynthetic genes are also expressed in the non-lignified cambium and in xylem parenchyma cells surrounding the lignifying TEs and fibres (Table 1; Fig. 3). During normal development, it is difficult to decipher if both lignifying and surrounding non-lignifying cells are producing lignin monomers. The expression of the genes for phenylalanine ammonia-lyase (PAL), C4H, 4-coumarate:CoA ligase (4CL), C3H, caffeoyl-CoA O-methyltransferase (CCoAOMT), cinnamyl alcohol dehydrogenase(CAD) and caffeic acid O-methyl transferase (COMT) also depends on the circadian/diurnal cycle (Rogers et al., 2005) and responds to biotic and abiotic stresses (Hawkins and Boudet, 2003; El Kayal et al., 2006; Fan et al., 2006; Kim et al., 2006; Hano et al., 2006; Yoshimura et al., 2008; Moura et al., 2010). As for many other genes, lignin biosynthetic genes are part of small multigenic families which sometimes show specificity for a distinct type of lignin. This is the case of At-CCR in A. thaliana which has two isozymes coded by different genes: At-CCR1 is preferentially expressed during development, and its loss-of-function mutants have reduced lignification (Jones et al., 2001), while At-CCR2 is expressed at a low level during development but is strongly induced during the defence reaction triggered by the pathogen Xanthomonas ssp. and could participate in formation of stressinduced lignin (Lauvergeat et al., 2001). The differential expression of genes coding for isoforms of enzymes in lignin monomer biosynthesis can represent one of the mechanisms contributing to the specific type of lignin deposited in distinct cell types: At-CCR1 during formation of developmental lignin and At-CCR2 during formation of pathogen stress-induced lignin Sub-cellular localization of related lignin monomer synthesis enzymes While the aromatic amino acid precursor(s) initiating lignin monomer synthesis derive from plastids, the enzymes implicated in lignin monomer synthesis are cytoplasmic and/or associate with the outer surface of the endoplasmic reticulum (ER) (Table 2). All the different studies which have investigated the sub-cellular localization of these enzymes are listed in Table 2 and summarized in Fig. 4. Based on these studies, the 1057 cytochrome P450 oxidoreductases responsible for the aromatic ring hydroxylation (C4H, C3H and F5H) are on the outer surface of the ER, whereas all the other lignin monomer biosynthetic enzymes (PAL, 4CL, CCoAOMT, CCR, CAD and COMT) are in the cytoplasm. However, a connection exists between the cytoplasm- and ER-localized enzymes as protein complexes can form between cytoplasmic PAL and ER-localized C4H (Achnine et al., 2004), as well as between all the ERlocalized cytochrome P450 oxidoreductases: C4H, C3H and F5H (Chen et al., 2011). Moreover, HCT (p-hydroxycinnamoyl-CoA:quinate/shikimate p-hydroxycinnamoyltransferase) and 4CL were found to be partially associated with the ER upon expression of C3H, suggesting an interaction between these proteins (Bassard et al., 2012). The oxido-reductive state of the different cytochrome P450 oxidoreductases, required for the hydroxylation of the aromatic ring, is maintained by the cytochrome P450 reductase 2 (ATR2) (Sundin et al., 2014). Thus, the synthesis of monolignols occurs in specialized sub-cellular areas at the interface between the cytoplasm and the outer surface of the ER (Fig. 4B). Lignin monomer transport mechanisms Although the synthesis of monolignols occurs within the cell protoplast, lignin deposition is restricted to the cell walls. Three types of transport mechanisms are suggested for the extracellular secretion of lignin monomers, namely passive diffusion (PD), vesicle-associated exocytosis and active ATPdependent transport using ABC transporters and/or proton coupled antiporters (Fig. 5). The ATP-dependent transport mechanism using ABC-G transporters has been identified for p-coumaryl alcohol (H-unit) export (Miao and Liu, 2010; Alejandro et al., 2012). However, the inhibition of ABC transporters in different xylem tissues (poplar, hybrid poplar, Japanese cypress and pine) using vanadate did not affect the transport of coniferin, whereas proton gradient erasers markedly reduced the transport, suggesting an export via proton-coupled antiporters (Tsuyama et al., 2013). The passive diffusion is supported by in vitro observations of the partitioning of lignin monomers by immobilized liposomes and lipid bilayer discs (Boija and Johansson, 2006; Boija et al., 2007); however, this mechanism is considered unlikely to play a major role in the mobilization of monolignols (Miao and Liu, 2010). Vesicle-associated secretion of lignin monomers was initially supported by the labelling of ER- and Golgi-derived vesicles in xylem TEs when feeding with tritium-labelled phenylalanine and subsequent autoradiographical imaging using electron microscopy (Pickett-Heaps, 1968; Fujita and Harada, 1979; Takabe et al., 1985). Improved radiolabelling feeding and detection techniques have since allowed the observation that part of the label incorporation is due to proteins rather than monolignols (Kaneda et al., 2008). Exocytosis of lignin monomers by vesicles was pharmacologically tested in in vitro TE differentiating cell cultures from Zinnia elegans using the exocytosis inhibitor brefeldin A (Ito et al., 2004). These experiments revealed a reduction of secreted phenolics, although the impact on lignin accumulation was not estimated. The mechanisms ensuring lignin monomer biosynthesis and export to the lignifying sites within the cell wall are still unclear. Mostly in veins and midrib Mostly in veins and midrib Mostly veins and midrib Mostly in veins and midrib Not defined Not defined Not defined Veins and midrib Primary xylem of midrib No expression Not defined Not defined Xylem vessel Not defined Veins and midrib Not defined Not defined Not defined Not defined Not defined Veins and midrib No expression Whole leaf, veins and midrib Not defined Not defined Nicotiana tabacum Arabidopsis thaliana Arabidopsis thaliana Arabidopsis thaliana Loblolly pine Nicotiana tabacum Medicago sativa Arabidopsis thaliana Populus tremula alba Populus trichocarpa Populus tremula alba Populus tremuloides Fragaria ananassa Saccharum officinarum Arabidopsis thaliana Zea mays Nicotiana tabacum Saccharum officinarum Populus sieboldii grandidentata Medicago sativa Zea mays Arabidopsis thaliana Arabidopsis thaliana Populus trichocarpa Populus tremuloides Aecondary xylem Vascular bundles Cambium, ray parenchyma and XPs Cambium, ray parenchyma and XPs Vascular bundles Not defined Not defined XPs Phloem, xylem fibres and XPs Vascular bundles and interfascicular fibres Diff. xylem cells; XPs Xylem tissue developping xylem Xylem and phloem parenchyma Not defined Xylem and phloem parenchyma Xylem vascular bundle and interfascicular fibres Ray parenchyma and XPs xylem and phloem parenchyma XPs Xylem fibres, young ray parenchyma, cambium and xylem vessels Xylem and phloem parenchyma Vascular system XPs Columella root cap; central cylinder No expression Vascular system Not defined Not defined Not defined XPs Not defined Not defined Diff. xylem cells; XPs No expression Not defined XPs Xylem vessel Not defined Cambium Vascular system Vascular system in mature xylem zone Vascular system Vascualr cylinder Diff. xylem cells Not defined Not defined Not defined Xylem tissue Low expression Whole root Vascular bundles Not defined Vascular system Not defined Vascular tissue and epidermis Entire root except meristem and root tip Entire root except meristem Vascular system Not defined Not defined Epidermis; primary xylem; phloem fibres Not defined Not defined Root GUS GUS IL IL IL GUS IL IL IL IL GUS qRT-PCR ISH IL IL IL GUS GUS GFP/GUS IL IL IL GUS GUS GUS GUS IL GUS GUS and ISH IL GUS GUS GUS ISH GUS Li et al. (1999) Goujon et al. (2003) Li et al. (2001) Li et al. (2001) Ruelland et al. (2003) Quentin et al. (2009) Li et al. (2001) Ruelland et al. (2003) Kersey et al. (1999) Sato et al. (2009) Chen et al. (2000) Shi et al. (2010) Lacombe et al. (1997) Ruelland et al. (2003) Blanco-Portales et al. (2002) Ruelland et al. (2003) Sibout et al. (2003) Nair et al. (2002) Vanholme et al. (2013) Hoffmann et al. (2004) Kersey et al. (1999) Zhong et al. (2000) Do et al. (2007) Nair et al. (2002) Capellades et al. (1996) Hu et al. (1998) Hoffmann et al. (2004) Lee et al. (1995) Gui et al. (2011) Sato et al. (2009) Pesquet et al. (2013); Serk et al. (2015) Nair et al. (2002) Franke et al. (2000) Harding et al. (2002) Bell-Lelong et al. (1997) Ohl et al. (1990) Hsieh et al. (2010) Zhu et al. (1995) Sato et al. (2009) IL GUS IL GUS Gray-Mitsumune et al. (1999) Osakabe et al. (2006) Reference GUS GUS Technique XP, xylem parenchyma; GUS, b-glucuronidase; ISH, in situ hybridization; IL, immunolabelling; GFP, green fluorescent protein; qRT-PCR, quantitative real-time PCR. F5H COMT CCR CAD CSE CCoAOMT HCT C3H Nicotiana tabacum Populus tremuloides 4CL Arabidopsis thaliana Oryza sativa Populus sieboldii grandidentata Cambium, xylem parenchyma Vascular bundles Xylem vessel and XPs Phloem fibres; rays; xylem; cambium Diff. xylem cells Mostly phloem cells Not defined Epidermis and vascular bundles Xylem fibres, young ray parenchyma, cambium and xylem vessels Vascular bundles XPs; diff. xylem cells Veins and midrib Mostly veins and midrib Not defined Mostly in xylem of midrib Xylem of midrib No expression Mostly in veins and midrib Epidermis and vascular bundles Not defined Xylem and sclerified parenchyma Mostly veins and midrib Arabidopsis thaliana Epidermis; primary xylem; phloem fibres Xylem fibres, young ray parenchyma, cambium and xylem vessels Vascular system Vascular tissue and epidermis Vascular tissue and epidermis Xylem and phloem fibres Stem C4H Whole leaf except veins and midrib epidermis; primary xylem; phloem fibres Not defined Leaves Mostly veins and midrib Not defined Not defined Populus trichocarpa deltoides Populus sieboldii grandidentata Populus kitakamiensis Species Arabidopsis thaliana Bambusa oldhamii Oryza sativa PAL Protein TABLE 1. Tissue-specific localization of lignin monomer biosynthesis transcripts/proteins in different species found in the literature 1058 Barros et al. — The cell biology of lignification in higher plants Barros et al. — The cell biology of lignification in higher plants 1059 B A C D TEs XPs RPs XFs 6 PAL 83 83 67 100 4 C4H 50 100 25 50 5 4CL 100 80 60 60 1 HCT 100 100 100 100 2 C3H 100 100 1 CSE 100 100 100 100 100 100 67 6 CCoAOMT 50 100 1 CCR 100 100 100 100 5 CAD 40 80 60 20 7 COMT 57 100 71 57 1 F5H 100 100 100 100 FIG. 3. Cell-specific expression of lignin monomer synthesis transcripts and proteins. Stem cross-sections of (A) arabidopsis, (B) Brachypodium and (C) Populus indicating the different cell types included in the studies in (D). Blue arrow, tracheary elements (TEs); orange arrow, xylem parenchyma (XPs); red arrow, ray parenchyma (RPs); purple arrow, xylary fibres (XFs). Scale bars ¼ 100 lm. (D) Percentage of studies supporting cell-specific expression of lignin monomer biosynthesis genes in TEs, XPs, RPs or XFs. Numbers in the first column indicate the number of individual studies, respectively, for each gene. Lignin monomer plasticity The identification of 4-hydroxyphenyl propene alcohols as the typical lignin monomers essentially results from in vitro polymerization studies. Monolignols can be used in combination with phenoloxidases to produce dehydrogenation polymers (DHPs) which have similar characteristics to lignin (Brunow and Lundquist, 1980). Several recent studies, however, revealed that lignin synthesis is not only restricted to the three canonical lignin monomers but also incorporates other unusual phenylpropene molecules exhibiting other substitutions such as monolignol precursors (i.e. hydroxycinnamic acid and aldehydes), esters of monolignols (coniferyl and sinapyl acetate or coumarate) (Morreel et al., 2004; Grabber et al., 2010; Vanholme et al., 2010; Wilkerson et al., 2014) but also other phenolic residues such as the flavone tricin (Del Rı́o et al., 2012; Lan et al., 2015) (Fig. 2). Furthermore, the specific accumulation of dilignols and trilignols has been observed in methanol-extractable fractions of the apoplast of lignifying xylem tissues (Morreel et al., 2004) and from the extracellular medium of lignifying Zinnia TE cell cultures (Tokunaga et al., 2005). However, the question remains whether these compounds represent intermediates of lignin synthesis, distinct apoplastic precursors needed for lignin synthesis or unrelated phenolics. Purified phenolic compounds accumulating in the extracellular medium of TE differentiating Zinnia cell cultures (which are chemically different from monolignols) were able to restore fully the lignin reduction of TEs treated with inhibitors of PAL, thus supporting the large diversity of potential/actual lignin monomers (Tokunaga et al., 2005). Moreover, due to the potential toxicity and low water solubility of monolignols (Whetten and Sederoff, 1995; Boerjan et al., 2003), 4-O-glucosides represent one of the substitutions which could combine both reduced toxicity and higher water miscibility. These monolignol glucosides are unable to form lignin without the intervention of a b-glucosidase in vitro (Chapelle et al., 2012) but can be taken up by plants to be incorporated into lignin (Tsuji and Fukushima, 2004) and are specifically transported by ATP-dependent transporters in the endomembrane system of lignifying woody tissues (Tsuyama et al., 2013). In addition, monolignol glucosides are highly accumulated in arabidopsis mutants which have extremely reduced Barros et al. — The cell biology of lignification in higher plants 1060 TABLE 2. Sub-cellular localization of related lignin monomer biosynthesis proteins in different species found in the literature Protein PAL C4H 4CL HCT C3H CCoAOMT CCR CAD COMT F5H Species Phaseolus vulgaris Primula kewensis Zinnia elegans Poplar kitakamiensis Populus sieboldii grandidentata Phyllanthus tenellus Poplar kitakamiensis Nicotiana tabacum Arabidopsis thaliana Nicotiana tabacum Populus sieboldii grandidentata Populus trichocarpa Nicotiana benthamiana Phaseolus vulgaris Poplar kitakamiensis Oryza sativa Nicotiana benthamiana Nicotiana benthamiana Nicotiana benthamiana Populus trichocarpa Medicago sativa Medicago sativa Zinnia elegans Zea mays Oryza sativa Zea mays Zinnia elegans Populus tremula alba Poplar euramericana, Eucalyptus globulus Zea mays Medicago sativa Poplar euramericana, Eucalyptus globulus Medicago sativa Medicago sativa Populus trichocarpa Tissue/cell type Localization Technique Reference Hypocotyl/XP, TE Head cells of glandular trichomes TE XP, fibre and vessels XP, fibre and vessels Cytosol ER Vesicles, PM and SCW Cytosol cytosol, ER, Golgi IL IL IL IL IL Smith et al. (1994) Schöpker et al. (1995) Nakashima et al. (1997) Takabe et al. (2001) Sato et al. (2004) Leaf mesophyl cell XP, TE Leaf epidermal cell Epidermal cells of hypocotyl, cotyledon and VND7-induced TEs Leaf epidermal cells XP, fibre and vessels Cytosol, chloroplast Plastid, cytosol Cytosol (col. C4H) ER IL IL FRET GFP fusion ER (col. PAL) Cytosol, ER, Golgi FRET IL Santiago et al. (2000) Osakabe et al. (2006) Achnine et al. (2004) Ro et al. (2001) and Schuetz et al. (2014) Achnine et al. (2004) Sato et al. (2004) Protoplast Leaves Hypocotyl/XP, TE Xylem fibre Protoplast Leaves Leaves Leaves Protoplast Stem/XP, TE Stem Developing TE Mesocotyl and root Protoplast Mesocotyl and root TE Stem GFP fusion FRAP IL IL GFP fusion FRAP FRAP FRAP GFP fusion IL Western IL Western GFP fusion Western, IL IL IL Chen et al. (2011) Bassard et al. (2012) Smith et al. (1994) Takabe et al. (2001) Rastogi et al. (2013) Bassard et al. (2012) Bassard et al. (2012) Bassard et al. (2012) Chen et al. (2011) Kersey et al. (1999) Guo et al. (2002) Ye (1997) Ruelland et al. (2003) Kawasaki et al. (2006) Ruelland et al. (2003) Nakashima et al. (1997) Šamaj et al. (1998) Xylem fibre ER (col. C4H) ER (col. C3H) ER Cytosol Cytosol cytosol (ER) Cytosol (ER) ER (col. C4H) ER (col. C3H) Cytosol Cytosol Cytosol Cytosol Cytosol Cytosol Vesicles, PM and SCW ER and Golgi-derived vesicles cytosol IL Takabe et al. (2001) Mesocotyl and root Stem/XP, TE differentiating xylem cells Cytosol Cytosol Cytosol Western, IL IL IL Ruelland et al. (2003) Kersey et al. (1999) Takeuchi et al. (2001) Stem Stem Differentiating xylem protoplasts Cytosol ER ER Western Western GFP fusion Guo et al. (2002) Guo et al. (2002) Wang et al. (2012) XP, xylem parenchyma; IL, immunolabelling; GFP, green fluorescent protein; FRET, fluorescence resonance energy transfer; FRAP, fluorescence recovery after photobleaching; col., co-localization with PAL/C4H/C3H; ER, endoplasmic reticulum; PM, plasma membrane; SCW, secondary cell wall. amounts of lignin (Zhao et al., 2013). However, the genetic loss-of-function mutants in UGT and BGLU, required for the addition and removal of the glucoside moiety, do not affect lignin accumulation or composition in arabidopsis plants (Lanot et al., 2006; Chapelle et al., 2012). In contrast to any other cell wall polymer, lignin appears to exhibit a tremendously high plasticity for its monomers. Lignin monomer production – autonomous or co-operative process The localization of the lignin monomer biosynthetic gene expression and their corresponding protein is present in the non-lignifying cells next to lignifying cells. For example, all the lignin monomer biosynthetic genes are expressed in both the actively lignifying interfascicular fibres of arabidopsis and the non-lignified meristematic cells of the cambium and in the associated parenchyma (xylem parenchyma during primary growth and ray cell files in secondary wood; Table 1; Fig. 3). This observation has led to the idea that cell wall lignification could proceed through cell co-operation where non-lignifying cells would provide the monomers and/or other substrates/enzymes to the cell wall of actively lignifying cells. This mechanism is referred to as ‘the good neighbour hypothesis’ or ‘the non-cell autonomous process’ (Smith et al., 2013; Pesquet et al., 2013). The lignification of distinct cell types can therefore be autonomous (a cell undergoing lignification produces enzymes and substrates for its lignification) and/or co-operative (neighbouring cells provide enzymes and/or substrates to lignifying cells). Co-operative lignin synthesis is clearly observed between TEs and fibres in the xylem of poplar trees as fibres directly neighbouring TEs present an intermediate lignin structure enriched in G-units like TEs, in contrast to their S-unit enrichment when only surrounded by fibres (Gorzsás et al., 2011). Although cell co-operation was hypothesized many years ago using the Zinnia TE differentiating cell Barros et al. — The cell biology of lignification in higher plants 1061 A Tyr Phe Nucleus Vacuole ER Monomer glucoside Common pathway H units Cytosol 8 PAL 6 C4H 3 4CL 1 HCT 2 C3H 3 CCoAOMT 2 CCR 4 CAD 4 COMT 2 F5H ER Cunits G units Golgi Plastids 40 Vesicles 20 13 Electrons S units Primary cell wall Plasma membrane Secondary cell wall B Monomer 7 PM 13 7 CW 7 75 7 13 75 25 100 100 100 100 25 13 13 25 13 13 100 100 FIG. 4. Sub-cellular localization of lignin monomer biosynthesis proteins. (A) Scheme illustrating the lignin monomer biosynthesis pathway and monomer transport in respect to sub-cellular localization of the lignin monomer synthesis proteins. Tyr, tyrosine; Phe, phenylalanine. (B) Percentage of studies supporting specific subcellular localization in: cytosol, endoplasmic reticulum (ER), Golgi, plastids, vesicles, plasma membrane (PM) or cell wall (CW). Numbers in the first column indicate the number of individual studies, respectively, for each protein. ATR2, arabidopsis cytochrome P450 reductase 2. culture system (Hosokawa et al., 2001), it cannot be generalized to all lignifying cell types. The Casparian strip of endodermal cells appears to depend on cell autonomous lignification (Alejandro et al., 2012), whereas xylem TEs appear to rely on a co-operative lignification with neighbouring xylem TE precursors and/or the surrounding parenchyma cells (Pesquet et al., 2013; Smith et al., 2013). The functional demonstration of TE non-cell-autonomous lignification was provided recently by several complementary studies including: (1) the pharmacological inhibition of lignification in Z. elegans in vitro TE differentiating cell cultures, which could be reverted when washing the inhibitor away once all TEs have committed cell death – which implies that the monolignols are supplied by the surrounding living cells – thereby demonstrating the cell co-operation during TE post-mortem lignification (Tokunaga et al., 2005; Pesquet et al., 2013); (2) arabidopsis plants knocked-out in xylem parenchyma-expressed genes implicated in co-operative lignification (i.e. At-RCD1 or At-MYB13) exhibited quantitative and qualitative changes in xylem lignin (Pesquet et al., 2013); Barros et al. — The cell biology of lignification in higher plants 1062 Monolignols O2 PD Monomer radical activation Monomeric coupling (with dirigent proteins) End-wise polymer extension LACCASE Vesicle DiP ABC trans. / PCA O2 NADPH NADPH O2 oxidase . O2 - DiP Redox Shuttle SOD Mn (II) Mn (III) Monomeric coupling NADP+ H+ Monolignols H2O2 PD Vesicle PEROXIDASE ABC trans. / PCA Monomer Symplast Plasma membrane Dimer Secondary cell wall Lignin polymer Primary cell wall FIG. 5. General lignin polymerization. Lignin monomers are exported across the plasma membrane either by passive diffusion (PD), by exocytosis (vesicle) or through ABC transporters and/or proton-coupled antiporter (PCA). Laccases and peroxidases activate the monomer radicals, resulting in the end-wise addition of and/or cross-reaction of the radical oligo/monomers with the extending polymer(s). Classical production of H2O2 and O2 derives from a two-step enzymatic process: NADPH oxidase and superoxide dismutase (SOD). Dirigent proteins (DiPs) stereospecifically restrict the radical coupling to one type of linkage. Manganese (Mn) acts as a redox shuttle to mediate radical activation (Onnerud et al., 2002). This schematic representation does not support equal importance or intervention of different possibilities used by specific cell types to form the lignin polymer. (3) specific silencing of the lignin monomer biosynthetic gene At-CCR1 in cells developing secondary cell walls using the IRX3/CesA7 promoter (specific to cells that develop secondary cell walls) did not completely abolish TE lignification, suggesting that thin-walled parenchyma contributes to TE lignification (Smith et al., 2013); and lastly (4) complementation of the lignin monomer biosynthetic gene At-C4H knock-out mutant with the promoter of master regulator VND6 (specific to metaxylem TE precursor cells) driving native C4H, which restored TE lignification, suggesting that thin-walled TE precursor cells might also contribute co-operatively to TE lignification (Yang et al., 2013). Similarly, the partial cell-autonomous lignification of xylem fibres was also demonstrated by specifically silencing At-CCR1 using the IRX3/CesA7 promoter, which reduces fibre lignin accumulation (Smith et al., 2013) and by complementing the At-C4H knock-out mutant with a VND6 promoter-driven C4H, which partially restores lignin accumulation in fibres (Yang et al., 2013). This suggests that fibre lignification is partially co-operating with the cambium and/or metaxylem TE cells. Altogether, cell-specific lignin deposition appears to result from distinct mechanisms depending on the cell type, from full cell autonomy to completely co-operative. However, the exact biological function and significance of these different levels of cell autonomy for lignification still needs to be fully understood. LIGNIN POLYMER FORMATION Compared with other cell wall polymers such as pectins and hemicelluloses, which are assembled remotely and exported to the cell wall, the formation of the lignin polymer occurs directly in the cell wall by the oxidative polymerization of secreted lignin monomers. The activated monolignol radicals present a relatively long half-life (approx. 45 s for phenoxy radicals; Harkin, 1967), partly stabilized by resonance, and are capable of diffusing through the wall to polymerize into lignin. Depending on both thermodynamical reactivity and steric accessibility, the different radical mesomers will assemble to form the different linkages of the polymer including both condensed C–C linkages such as 5–5’, b–5’, b–b and b–1’, and non-condensed C–O–C ether linkages such as b-O-4’ (Terashima and Fukushima, 1988). The diversity of the most prevalent intermonomeric linkages in different plant taxa is illustrated in Table 3. Both the polymer length and the proportion of different linkages can be explained by the availability and reactivity of specific lignin monomer(s) radicals (Ralph et al., 2008). The impact of monomer availability on the polymer length is illustrated during in vitro DHP formation, which increases in size when supplied continuously with new monomers compared with when supplied with an initial bulk (Tanahashi and Barros et al. — The cell biology of lignification in higher plants 1063 TABLE 3. Percentages of the different interunit linkages in lignin of different plant taxa Species Sample b–O-4 5–5 b–5 b–1 b–b Reference Spruce Pine Birch Poplar Arabidopsis MMW MMW MMW Wood Stem Alfalfa Maize Stem Stem Wheat Straw 48 62 60 69 79 77 81 100 60 75 10 – 5 – – 1 1 – – 3 10 20 6 3 7 15 8 – 27 11 7 – 7 – – 1 – – 3 3 2 18 3 28 14 6 6 – 10 4 Adler (1977) Mansfield et al. (2012) Adler (1977) Mansfield et al. (2012) Mansfield et al. (2012) Bonawitz et al. (2014) Marita et al. (2003) Mansfield et al. (2012) Min et al. (2014) Del Rı́o et al. (2012) b–O-4, beta-aryl ether; 5–5, biphenyl and dibenzodioxocin; b–5, phenylcoumaran; b–1, 1,2-diaryl propane; b–b, resinol; MMW, mature milled wood. Higuchi, 1981). The proportion of the different linkages in the polymer depends on the available monomer type: for example in the case of knock-out/down mutation of the COMT gene (Fig. 2), more of the normally rare lignin sub-structure benzodioxane is detected in arabidopsis, Medicago and Populus (Lu et al., 2010; Moinuddin et al., 2010) due to the increased presence of the unconventional 5-hydroxyconiferyl alcohol. Unlike cellulose, in which the addition of new monomers to the extending polymer occurs directly by a polymerizing enzyme, lignin end-wise addition to extend the polymer with radical oligo/monomers occurs independently of processing enzymes (Fig. 5). The mono/oligolignol radicals are produced enzymatically by two kinds of phenol-oxidoreductase enzymes, O2dependent laccases and H2O2-dependent peroxidases, which have both been shown to enable monolignols to polymerize into DHPs in vitro (Sterjiades et al., 1992, 1993). Laccase-catalysed lignin polymer formation Plant laccases (EC 1.10.3.2), or blue copper-containing oxidoreductases, are part of a medium size multigenic family (17 genes in arabidopsis and 39 genes in Populus trichocarpa; Weng and Chapple, 2010) with an N-terminal peptide signal guiding the protein through the secretory pathway. Laccases can act as p-diphenol:O2 oxidoreductases by using O2 directly to oxidize all types of monolignols to form DHPs (Higuchi and Ito, 1958; Sterjiades et al., 1992; Bao et al., 1993; Ranocha et al., 1999; Kärkönen et al., 2002; Liang et al., 2006). Laccases do not appear to have specifically evolved to enable plant lignin polymer formation and present a high degree of structural conservation between bacteria, fungi and plants (Dwivedi et al., 2011). The number of laccases has slightly increased, ranging during plant evolution from three in Chlamydomonas reinhardtii, to 12 in Physcomitrella patens, ten in Selaginella moellendorffii and 17–39 in angiosperms (Weng and Chapple, 2010). Interestingly, laccases in saprophytic fungi exhibit lignolytic activity and catalyse wood lignin disassembly (Coy et al., 2010; Crestini et al., 2010). The diversity/redundancy of laccase function in plants is shown by multiple minor phenotypic defects in single loss-of-function mutants in arabidopsis laccases: they show minor changes such as a higher susceptibility to polyethylene glycol (PEG)-induced dehydration for laccase-2, early flowering for laccase-8 or pale seed colour for laccase-15 (Cai et al., 2006). The experimental evidence for the intervention of laccases during lignification is supported by (1) their specific transcription profiles – laccases are co-regulated with lignin monomer biosynthesis genes (Gavnholt and Larsen, 2002), with secondary cell wall-forming genes (Brown et al., 2005) and expressed in lignifying tissues in arabidopsis (Berthet et al., 2011), and (2) their enzymatic activity: laccase activities were detected during xylem lignification in several species (Dean and Eriksson, 1994; Liu et al., 1994; Richardson and McDougall, 1997; Dean et al., 1998; Ranocha et al., 1999; Kärkönen et al., 2002; Ranocha et al., 2002; Caparrós-Ruiz et al., 2006; Koutaniemi et al., 2015). Functional studies have now confirmed the role of specific laccase isoforms during the lignification of specific cell types (Tables 4 and 5). In arabidopsis, laccase-15 (At-LAC15) is responsible for seed coat lignification, and the loss-of-function mutant (named transparent testa 10 because of reduced seed coat brownish colour) exhibits a 30 % decrease in seed lignin content (Liang et al., 2006). The At-LAC15 mutant has a significantly reduced capacity to oxidize coniferyl alcohol and an increased presence of b–b/b–5 and b–O-4 linkages in seed cells compared with wildtype seeds (Liang et al., 2006). Arabidopsis laccases-4, -11 and -17 are responsible for the lignification of both xylem TEs and fibres (Berthet et al., 2011; Zhao et al., 2013). Single and double loss-of-function mutants in these laccases show a subtle effect on stem growth in normal conditions, but double mutants present a significant reduction in G-type lignin (Berthet et al., 2011) (Table 5). The triple mutant exhibits an extremely reduced growth and a dramatic reduction in xylem lignin, suggesting that xylem lignification results from the combined activity of these three laccases (Zhao et al., 2013) (Table 5). Arabidopsis laccase-17 (At-LAC17) is localized in the secondary cell walls of xylem vessels and stem interfascicular fibres, as well as xylem parenchyma, and vascular and interfascicular cambium, while At-LAC4 is present in cambium, xylem parenchyma and interfascicular fibres, visible in both the compound middle lamella and the secondary cell wall of xylem vessels and fibres (Berthet et al., 2011, Schuetz et al., 2014) (Table 4). Similarly to arabidopsis, poplar xylem lignification also depends on multiple laccase isoforms. The downregulation of a single wood-expressed laccase using constitutive antisense constructs did not reduce lignin quantity or composition but interestingly altered the cell wall structure of xylem fibres (Ranocha et al., 2002) whereas the general silencing of poplar laccases using the constitutive overexpression of microRNA397a – which targets Barros et al. — The cell biology of lignification in higher plants 1064 TABLE 4. Tissue-/cell-specific expression and impact on growth and vessel morphology of mutations in different peroxidases and laccases associated with lignification in Arabidopsis thaliana plants Protein AT - number Expressed in tissue/cell type Modulation Growth Vessel Lac4 At2g38080 Cambium, xylem parenchyma, interf. fibres KO No effect IRX Lac11 At5g03260 KO No effect – Lac15 At5g48100 KO Shorter root – Lac17 At5g60020 KO No effect Slight IRX Lac4/Lac17 Lac4/Lac11 Lac11/Lac17 Lac4/Lac11/ Lac17 Prx2 Prx4 Prx25 Prx37 Prx47 Prx52 Prx53 Prx64 – – – – At1g05250 At1g14540 At2g41480 At4g08770 At4g33420 At5g05340 At5g06720 At5g42180 KO KO KO KO KO KO KO 35S OE – KO – KD No effect No effect No effect Dwarf No effect No effect No effect Shorter – No effect – – IRX – – – No effect No effect No effect No effect – No effect – – Prx66 At5g51890 Xylem parenchyma, vascular and interf. cambium Seed coat, xylem parenchyma, vascular and interf. cambium Xylem vessels and parenchyma, interf. cambium and fibres, vascular cambium – – – – – – – Vascular bundle Endodermis, xylem parenchyma – Vascular bundle Cortex, epidermis, endodermis, xylem fibres Xylem vessels – – – Prx71 Prx72 Prx2/Prx25 Prx2/Prx71 Prx25/Prx71 At5g64120 At5g66390 – – – – – – – – KO KO KO KO KO No effect Shorter No effect No effect No effect No effect IRX No effect No effect No effect Reference Berthet et al. (2011); Turlapati et al. (2011); Zhao et al. (2013) Turlapati et al. (2011); Zhao et al. (2013) Liang et al. (2006); Turlapati et al. (2011) Berthet et al. (2011); Turlapati et al. (2011) Berthet et al. (2011) Zhao et al. (2013) Zhao et al. (2013) Zhao et al. (2013) Shigeto et al. (2013) Fernández-Pérez et al. (2015) Shigeto et al. (2013) Pedreira et al. (2010) Tokunaga et al. (2009) Fernández-Pérez et al. (2014) Østergaard et al. (2000) Tokunaga et al. (2009); Lee et al. (2013) Sato et al. (2006); Tokunaga et al. (2009) Shigeto et al. (2013) Herrero et al. (2013) Shigeto et al. (2015) Shigeto et al. (2015) Shigeto et al. (2015) KO, knock-out mutant; KD, knock-down; OE, overexpressor; interf., interfascicular; IRX, irregular xylem phenotype (collapsed vessels). 29 out of the 47 poplar laccases – induces significant reduction of wood lignin in poplar (Lu et al., 2013). Peroxidase-catalysed lignin polymer formation Plant peroxidases, mostly represented by class III type peroxidases, are part of a large multigenic family (73 genes in arabidopsis) with a peptide signal that targets the proteins through the secretory pathway (Hiraga et al., 2001; Valério et al., 2004). In contrast to laccases, the number of peroxidase isoforms has increased tremendously during plant evolution, ranging from none in C. reinhardtii, to 43 in the non-lignified moss P. patens, 79 in S. moellendorffii and 73–138 in angiosperms (Weng and Chapple, 2010). Plant class III peroxidases (EC 1.11.1.7) have been shown to catalyse the formation of DHPs efficiently in vitro using H2O2 and monolignols (Sterjiades et al., 1993; Guerra et al., 2000). The enzymatic activity of peroxidases to produce DHPs appears to be more specific to coniferyl alcohol and peroxidases appear to be unable to use sinapyl alcohol as a substrate (At-PRX53, Østergaard et al., 2000; AtPRX34, Demont-Caulet et al., 2010). However, some purified peroxidases from other plant species (poplar and silver birch) have shown affinity for sinapyl alcohol (Tsutsumi et al., 1998; Fagerstedt et al., 2010). Like laccases, peroxidases are expressed in lignifying vascular tissue (Table 4). In TE differentiating cell cultures of Zinnia, the homologous protein to At-PRX66 is localized in TE secondary cell walls and its recombinant protein showed a strong preference for coniferyl alcohol (Sato et al., 2006). Like laccases, the active role of peroxidases during lignin formation was shown by reverse genetic approaches in multiple species: the downregulation of PRX60 in tobacco plants (Blee et al., 2003) or of PRX3 in aspen (Li et al., 2003) resulted in phenotypes which had reduced lignin accumulation and altered lignin composition. In A. thaliana, endodermal Casparian strip lignification was significantly reduced when silencing At-PRX64 in the root endodermis (Lee et al., 2013). Moreover, arabidopsis single or double knock-out mutants in At-PRX2, At-PRX71 and At-PRX25 exhibited reduced lignin accumulation without affecting stem height (Shigeto et al., 2013, 2015), and the mutation of At-PRX72 caused a reduction in lignin and stem height (Herrero et al., 2013) (Tables 4 and 5). Second substrate requirement – H2O2 and/or O2 – during lignin polymer formation Besides the lignin monomer, peroxidases and laccases require additional substrates – hydrogen peroxide (H2O2) and molecular oxygen (O2), respectively – to form monolignol radicals (Fig. 5). Limiting concentrations of O2 are unlikely in normal growth conditions as O2 is present in large proportion in the air and is transported in the xylem sap. Gansert (2003) suggests that O2 contributes to 70 % of xylem oxygenation in birch. However, during anoxia/hypoxia, the triggered arrest of plant Barros et al. — The cell biology of lignification in higher plants 1065 TABLE 5. Impact on total lignin content and H-, G-, and S-unit composition of mutations in different peroxidases and laccases associated with lignification in Arabidopsis thaliana plants Protein Lac4 Lac11 Lac15 Lac17 Lac4/Lac17 Lac4/Lac11 Lac11/Lac17 Lac4/Lac11/Lac17 Prx2 Prx4 Prx25 Prx37 Prx52 Prx64 Prx66 Prx71 Prx72 Prx2/Prx25 Prx2/Prx71 Prx25/Prx71 Modulation KO KO KO KO KO KO KO KO KO KO KO 35S OE KO KD – KO KO KO KO KO Total lignin H-units G-units S-units S/G ratio ; No effect ; ; ; ; ; ; ; ; ; – ; – – No effect ; ; ; ; No effect No effect – No effect No effect No effect No effect – – – – – – – – – : No effect No effect No effect ; No effect – ; ; ; ; – No effect : : – : – – : – : : : No effect No effect – No effect – ; ; – : ; : – ; – – : – : : : ;vb :if – – ;vb :if – – – – : ; : – ; – – : No effect : : : :, increase; ;, decrease in total lignin; KO, knock-out mutant; KD, knock-down; OE, overexpressor; vb, vascular bundle; if, interfascicular fibres. References for each study are provided in Table 4. growth is coupled to an increase of H2O2 (Blokhina et al., 2001) as well as an increase of lignin in both the Casparian strip and the sclerenchyma cells in rice (Kotula et al., 2009). The pharmacological scavenging of H2O2, using KI or catalase, clearly reduces the lignification of the Casparian strip (Lee et al., 2013), the co-operative lignin formation in TEs (Pesquet et al., 2013) and the extracellular lignin accumulation in Picea abies cell cultures (Kärkönen et al., 2002). Similarly to other reactive oxygen species (ROS), H2O2 molecules have extremely short half-lives ranging from 109 s to 1 ms (D’Autréaux and Toledano, 2007) and are thus unable to accumulate, which suggests that they are constantly produced. The production of apoplastic H2O2 derives from a two-step enzymatic process using nicotinamide adenine dinucleotide phosphate hydrogen oxidase (NADPH oxidase) and superoxide dismutase (Ogawa et al., 1997). NADPH oxidase NADPH oxidases, also called Respiratory Burst Oxidase Homolog (RBOH) proteins, are plasma membrane proteins with six conserved transmembrane domains and cytosolic FAD- and NADPH-binding sites, which catalyse the production of apoplastic superoxide (O2–) from cytoplasmic NADPH (Sagi and Fluhr, 2006). In arabidopsis, NADPH oxidases are part of a small multigenic family of ten isoforms labelled from A to J, which are expressed in different organs and in response to both plant development and defence (Torres et al., 2006; Sagi and Fluhr, 2006; Lee et al., 2013). In rice, the synthesis of stress-induced lignin by pathogen attack is mediated by the Rac/Rop small GTPases RAC1 which binds to CCR1 (Kawasaki et al., 2006) and activates NAPDH oxidase (Kawasaki et al., 1999). Similarly, xylem parenchyma cells have been shown to express specifically Rac small GTPase (Nakanomyo et al., 2002) and produce O2–/H2O2 in a polarized way, only on the cell side in contact with TEs (Ros Barceló, 1998, 2005). The functional association of NADPH oxidase with lignification was unravelled using both pharmacological inhibition by diphenyleneiodonium (DPI) and genetic analysis using loss-of-function mutants. DPI treatment prevents the lignification of the endodermal Casparian strip (Lee et al., 2013) and reduces the co-operative lignification of Zinnia TE cell cultures (Pesquet et al., 2013). Mutations in NADPH oxidase RBOH-F (AT1G64060) deplete the endodermal Casparian strip of lignin without apparently affecting TE lignification (Lee et al., 2013). Moreover, mutants in xylem parenchymaexpressed At-RCD1 (Radical-Induced Cell Death1; Overmyer et al., 2000) present an elevated amount of ROS as well as an increase of lignin in the xylem (Pesquet et al., 2013). The AtRCD1 mutant phenotype can be partially compensated when mutating NADPH oxidase isoforms D and F (Zhu et al., 2013), suggesting that these enzymes control the appropriate supply of ROS for lignification. Similarly to the cell-specific expression of different phenoloxidase isoforms, distinct NADPH oxidase isoforms appear to be associated with the lignification of specific cell types. High isoelectric point superoxide dismutase (SOD) Superoxide dismutases (EC 1.15.1.1), divided into three different forms (CuZn-SOD, Fe-SOD and Mn-SOD), catalyse the dismutation of toxic superoxide radicals produced by NADPH oxidase into O2 and H2O2 substrates which can be used by laccases or peroxidases, respectively (Liochev and Fridovich, 1994). Superoxide dismutation by CuZn-SOD (Karpinska et al., 2001) was confirmed pharmacologically to occur in lignifying xylem tissues of spinach (Ogawa et al., 1997) and showed distinct subcellular localization in the secondary cell walls of 1066 Barros et al. — The cell biology of lignification in higher plants xylem TEs and fibres, as well as in the middle lamella of surrounding xylem parenchyma cells in scots pine, poplar and Zinnia TEs (Karpinska et al., 2001; Karlsson et al., 2005; Srivastava et al., 2007). Pharmacological inhibition of SOD reduced Zinnia TE H2O2 production as well as TE lignin accumulation (Karlsson et al., 2005) and similarly reduced the lignification of the endodermal Casparian strip (Lee et al., 2013). Nevertheless, genetic modulation of CuZn-SOD using antisense constructs in transgenic poplar reduced the growth rate but only slightly reduced lignin accumulation (Srivastava et al., 2007). Interestingly, constitutive overexpression of cytoplasmic SOD in arabidopsis had the opposite effect and improved performance of plant growth under salt stress due to an increase in secondary cell wall synthesis and possibly lignin accumulation (Gill and Tuteja, 2010). Overall, these results suggest that SODs are, at least partially, involved in the lignification of different tissues. lignification (Zhao et al., 2013), suggesting that laccases and peroxidases do not function redundantly. However, this cell specificity for phenoloxidases is not so clear-cut as xylem TE lignification requires both laccases and peroxidases (Table 5), and collapsed TE phenotypes have been observed in lossof-function mutants of both At-LAC4 (Brown et al., 2005; Berthet et al., 2011) and At-PRX72 (Herrero et al., 2013). This led to the idea that laccases and peroxidases could act in a sequential order during cell lignification (Sterjades et al., 1993), starting with laccases and followed by peroxidases, to accomplish full cell wall lignification. The late intervention of peroxidases was further supported as they can form rigid cross-links between lignin, hemicelluloses and extensins in the secondary cell wall (Mäder et al., 1977, 1980; Lamport, 1986; Lagrimini et al., 1987; Passardi et al., 2004) once the initial lignin polymers/oligomers are made by laccases. Three hypothetical models could therefore explain this dual intervention of laccases and peroxidases during cell wall lignification. Intermonomeric linkage and dirigent proteins Model of sequential intervention due to different substrate specificity. In this model, the radical activation of the mono- The mechanisms controlling the proportion of different intermonomeric linkages within the lignin polymer in the different parts of specific cell types is still poorly understood. Each specific linkage has been considered to result from differences in thermodynamic reactivity of the monomer radical resonance forms (Watts et al., 2011) but have also been suggested to depend on specific proteins, named dirigent proteins (DiPs) (Fig. 5). DiPs govern the stereochemistry of the oxidative coupling of coniferyl alcohol catalysed by both peroxidases and laccases, leading to the production of a less thermodynamically favourable b–b linkage compared with b–O-4 [observed during (neo)lignan(s) synthesis; Halls and Lewis, 2002]. DiP genes have been found to be expressed during xylem lignification, and their corresponding proteins are localized in secondary cell walls of cells undergoing lignification (Kwon et al., 1999). Functional evidence of the intervention of DiP in lignification was provided by the loss-of-function mutant of the DiP enhanced suberin 1 (ESB1, AT2G28670) which exhibited both an increased lignin deposition and a misorganization of lignin in the endodermal Casparian strip (Hosmani et al., 2013). The biological control of intermonomeric linkages by DiPs could explain the structural difference observed between DHPs and native lignins (Davin and Lewis, 2000). The extent to which random chemical coupling compared with DiP-dependent coupling contributes to the cell-specific lignin polymer formation remains, however, unknown. Lignin polymerization: laccase and peroxidase? Although both laccases and peroxidases can catalyse the formation of monolignol radicals and form DHPs in vitro, these enzymes appear to be differently implicated in the cell wall lignification of specific cell types. In A. thaliana, the Casparian strip lignification depends on peroxidases (essentially AtPRX64; Lee et al., 2013) whereas xylem TE and fibre lignification are mostly dependent on laccases (essentially At-LAC4, At-LAC11 and At-LAC17; Zhao et al., 2013). Interestingly, the laccase triple knock-out mutant which has severely reduced xylem lignification is not affected in its Casparian strip mer(s) first requires laccases to produce the initiating oligolignols and/or the core lignin polymers which are then subjected to peroxidases for further branching and/or extension. This hypothesis relies on the capacity of peroxidases and laccases to oxidize distinct substrates, other than monolignols, as shown by histological detection of phenoloxidase activities in lignifying tissue: 4-methoxy-a-naphthol reveals the presence of peroxidases but not laccases, whereas ABTS [2,2-azinobis-(3-ethylbenzothiazoline-6-sulphonate)] and coniferaldehyde show both peroxidase and laccase activities in lignifying poplar (De Pinto and Ros Barceló, 1997; Ranocha et al., 1999). Thus, ligninassociated laccases and peroxidases would catalyse the radical activation of distinct molecules produced sequentially during the end-wise polymerization of lignin. Model of sequential intervention due to different spatio-temporal expression. In this model phenoloxidase enzymes are differen- tially localized/secreted within the TE primary and secondary cell wall. The localization of laccase and peroxidase gene expression is found in both lignifying and non-lignifying xylem cells, suggesting both cell-autonomous and co-operative processes (Table 5). Moreover, differences in the spatio-temporal expression of specific phenoloxidase during tissue lignification could explain the sequential intervention of laccases and peroxidases. Model of differential protein complex formation. In this model, phenoloxidases are associated together with other proteins (DiPs or others) forming protein complexes restricting substrate specificity and/or localization in the cell wall. This model is supported by the laccase/cellobiose dehydrogenase interaction in Pycnoporus cinnabarinus fungus which specifically enables the formation of the phenol-derived pigment cinnabarinic acid (Temp and Eggert, 1999). Moreover, a multicomponent protein complex containing manganese (II)-dependent peroxidase, laccase and b-glucosidase was found in the fungus Lentinula edodes which was able to transform pentachlorophenol and 2,5dichlorophenol (Makkar et al., 2001). Such protein complexes could form at a specific time and/or specific sites to enable specific steps required for lignification. Barros et al. — The cell biology of lignification in higher plants Together, these models aim to explain the mechanisms leading to differential lignin accumulation, size and composition in distinct sub-domains of the cell wall (middle lamella vs. secondary cell wall) of specific cell types (xylem TEs vs. fibres), although currently our understanding is still largely fragmentary. LIGNIN FORMATION DURING THE DIFFERENTIATION OF SPECIFIC CELLS Lignification is an integral part of the differentiation process of several specialized cell types to fulfil their physiological function. However for each distinct cell type, lignin deposition exhibits differences in (1) its timing during the cell differentiation process (before and/or after cell death), (2) its dependency on specific enzymes and/or substrates, (3) its sub-cellular deposition in the cell wall, (4) its monomeric composition and (5) its autonomy for the production of the required enzymes and/or substrates. This complexity is illustrated by the multiple phenotypic defects observed in lignin synthesis knock-out mutants in A. thaliana which have altered lignification of seeds (Liang et al., 2006), reduced height with a lower lignification of xylem fibres (Smith et al., 2013), defects in xylem TE resistance during sap conduction (Jones et al., 2001) and/or increased susceptibility to root pathogen infection (Cahill and Mc Comb, 1992; Wuyts et al., 2007). It is therefore difficult to generalize lignification to one common cellular mechanism and, in order to provide a better picture of the different deposition mechanisms, the lignification processes during the differentiation of three distinct cell types are described below. Lignification of xylem tracheary elements The vascular and mechanical support functions of TEs is established by the removal of the cell cytoplasm by programmed cell death and the reinforcement of the TE cell side walls with a lignified secondary cell wall (Pesquet and Lloyd, 2011). TE differentiation from the elongated cells of the meristematic cambium progresses sequentially with (1) a microtubule-guided cellulose and xylan secondary cell wall deposition (Oda et al., 2010; Pesquet et al., 2010), which is followed by (2) the cellautonomous programmed cell death, and concluded by (3) the post-mortem lignification of the TE secondary cell wall and the autolysis of the cell content (Fig. 6A). In primary tissues, TE lignin deposition is restricted to the secondary cell wall thickenings and it is completely absent from the modified TE primary cell walls in TEs of Coleus (Hepler et al., 1970), Zinnia (Taylor et al., 1992) and arabidopsis (Schuetz et al., 2014; Serk et al., 2015). On the other hand, in secondary tissues, TE lignin deposition is present in both secondary and primary cell walls (Fromm et al., 2003; Gierlinger, 2014; Herbette et al., 2015; Serk et al., 2015). Interestingly, the lignification of TEs in secondary tissues initiates at the cell corner of the middle lamella and then gradually progresses through the secondary cell wall layers (Terashima and Fukushima, 1988, 1989; Donaldsson et al., 1999). The initiation of TE lignification before or after TE cell death is still debated, although all agree that TE lignin deposition will 1067 continue once TE cells have died (Pesquet et al., 2013; Smith et al., 2013). This post-mortem lignification of TEs operates by the co-operative supply of lignin monomers and ROS by both the surrounding precursor cells and xylem parenchyma (Ros Barceló et al., 2005; Pesquet et al., 2013). The main enzymes associated with TE lignin oxidative polymerization are AtLAC4 (Brown et al., 2005) and At-PRX72 (Herrero et al., 2013), as both single loss-of-function mutants have collapsed TE cell walls. This defect is due to altered secondary cell wall structures observed also when reducing lignin biosynthesis in plants either pharmacologically or genetically such as when AtCCR1 is mutated in IRX4 (Jones et al., 2001). Based on the expression of other phenoloxidases in cells surrounding TEs (Table 4), At-LAC4 and At-PRX72 probably act with other phenoloxidases produced in both TEs and xylem parenchyma (Berthet et al., 2011; Zhao et al., 2013). The biological significance of TE post-mortem lignification has not been yet unravelled, but one hypothesis is that TEspecific non-cell-autonomous lignification is a mechanism to enable optimal hydro-mineral sap flow without renewing the entire vascular system (Ménard and Pesquet, 2015). As the plant increases in height and width, the tension pressure that is necessary to enable sap rising as well as the pressure within the stem tissues increases. Post-mortem lignification of TEs might have evolved to withstand the increasing pressure in the stem by prolonged lignification of the TEs along with the increase in height and girth of the stem. Lignification of xylem fibres Sclerenchyma fibres are believed to derive from a primitive ancestral xylem cell which evolved into TEs mainly for the sap conduction and fibres for mechanical support (Lucas et al., 2013). In contrast to TEs, fibres do not require cell death to achieve their structural support function. Sclerenchyma fibre cells derive from the xylem meristem and differentiate as they elongate by tip growth with a microtubule-dependent deposition of xylan and cellulose in the secondary cell wall (Chaffey et al., 2002) which is then impregnated with lignin, starting at the cell corner of the compound middle lamella and progressing gradually inwards towards the innermost layer of the secondary cell wall (Donaldson, 2001) (Fig. 6B). Although both TEs and fibres develop thick lignified secondary cell walls, xylem fibres present a lignin enriched in S-units. Unlike TEs, xylem sclerenchyma fibres in arabidopsis are mostly lignified by cellautonomous processes. Reduced lignin accumulation in fibres is observed in both the loss-of-function mutants in the lignin monomer biosynthesis gene At-CCR1 (Jones et al., 2001) and in plants silencing At-CCR1 using artificial microRNA driven by the secondary cellulose synthase promoter IRX3 (Smith et al., 2013). However partial co-operative lignification of xylem fibres is also observed with the neighbouring cells: poplar xylem fibres next to TEs exhibit a lignin composition intermediate between that of TEs and fibres with a polar enrichment in G-units (Gorzsás et al., 2011). Lignin oxidative polymerization in sclerenchyma fibres depends on multiple laccases (Berthet et al., 2011; Lu et al., 2013; Zhao et al., 2013) as well as peroxidases (Blee et al., 2003; Herrero et al., 2013; Shigeto et al., 2013) (Tables 4 and 5). Barros et al. — The cell biology of lignification in higher plants 1068 A Co-operative lignification (tracheary elements) Tracheary element (alive) - hemi-/cellulose deposition - Cambial cell Neighbouring xylem/ray parenchyma cell Tracheary element (dead) - lignin deposition - Monolignols Monolignols PCD Differentiation H2O2 LAC/PRX Lac17/Prx 72 LAC/PRX Lac4/Prx72 . O O2 B O2 2 Partial co-operative lignification (xylem fibres) Xylem fibre - Lignin deposition - Xylem fibre - hemi-/cellulose deposition - Cambial cell Monolignols Differentiation Neighbouring xylem/ray parenchyma cell Monolignols Monolignols Lignification LAC/PRX ROS LAC/PRX ROS C Autonomous lignification (casparian strip) CASP domain formation Localization of NADPH oxidase and SOD Localization of ESB1 Export of monolignolsand peroxidases for lignification Monolignols O2 . O2- .O 2 PRX Prx64 .O 2 H2O2 H2O2 PRX Prx64 Monolignols NADPH oxidase Plasma membrane CASP Primary cell wall Peroxidase SOD ESB1 Non-lignified secondary cell wall ABCG transporter Lignified secondary cell wall/ apoplastic space FIG. 6. Different levels of co-operative and autonomous lignification depending on the cell types studied: xylem tracheary elements, xylem fibres and endodermal cells. (A) Different stages of TE formation showing post-mortem TE lignification through neighbouring parenchyma cells that provide monolignols, laccases/ peroxidases (LAC/PRX) and O2/H2O2 produced by NADPH oxidase and superoxide dismutase (SOD). Living neighbouring TEs may also provide monolignols and incorporate LAC/PRX in the secondary cell wall before programmed cell death (PCD). (B) Differentiation and partial co-operative lignification of xylem fibres showing that monolignols, LAC/PRX and reactive oxygen species (ROS) are produced by both xylem fibres and neighbouring parenchyma cells. Living unlignified fibres may also provide monolignols. (C) Autonomous lignification in endodermal cells during Casparian strip formation, showing the formation of the Casparian strip domain by CASP proteins (Casparian strip membrane domain proteins) and the localization of ESB1 (enhanced suberin 1) in the Casparian strip zone. Later on, H2O2 (produced by NADPH oxidase and SOD), monolignols (exported by ABCG transporters) and PRX are supplied to the Casparian strip zone. Barros et al. — The cell biology of lignification in higher plants Lignification of root endodermal cells Endodermal cells constitute a tightly bound root cell layer isolating and regulating the flux of compounds/nutrients from the root cortex in contact with the rhizosphere to the root medullar vascular system (Geldner, 2013). This barrier property is directly due to the apoplastic polarized deposition of a lignified and suberized Casparian strip which frames and radially interconnects each endodermal cell (Fig. 1). Lignin deposition in this cell type is achieved independently of cell death and is necessary to establish the apoplastic barrier (Naseer et al., 2012). The differentiation of these cells derives from the root tip and, once cells have fully elongated, the Casparian strip is formed (Fig. 6C) by: (1) establishing specific polarized domains, named CASPs (Casparian strip membrane domain proteins), which will delimit the future sites of the Casparian strips both at the plasma membrane (localization of At-CASP1; Roppolo et al., 2011) and in the neighbouring apoplastic space (localization of ESB1; Hosmani et al., 2013); (2) NADPH oxidase RBOH-F is targeted to the CASP domain and At-PRX64 is exported into the CASP- delimited apoplastic space (Lee et al., 2013); (3) lignin monomers are secreted in the apoplast, some by an unpolarized ABC transporter such as ABCG29 (Alejandro et al., 2012); finally, (4) lignin is polymerized in the apoplastic space delimited by the CASP domain using peroxidases, the apoplastic monomers and H2O2 (from O2– generated by RBOH-F and converted by an unidentified apoplastic SOD). CONCLUSIONS Lignin is associated with many different specialized cells to fulfil specific physiological functions, but exhibits distinct properties for each cell type, which may explain why no general mechanism for lignification has been yet defined. Thus depending on the desired lignin properties for the cell function, specific substrates and enzymes will be produced in distinct cell types although a high plasticity for both substrates and enzymes will still be retained. Depending on the different cell types, lignification will progress autonomously and/or co-operate with the surrounding cells to ensure full lignification and to adapt to environmental changes. ACKNOWLEDGEMENTS The authors would like to acknowledge the memory of Barek Tamasloukht. The authors thank Professor Leszek A. Kleczkowski, Drs Emiko Muruzoka and Raphael Decou, Mrs Delphine Ménard and Mr Charilaos Dimotakis for critical comments. This research was supported by Vetenskapsrådet (VR) research grant 2010-4620 (to E.P.), the Gunnar Öquist Fellowship from the Kempe Foundation (to E.P.) and the Carl Trygger Foundation (to E.P.). LITERATURE CITED Achnine DL, Blancaflor EB, Rasmussen S, Dixon RA. 2004. Colocalization of L-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channeling in phenylpropanoid biosynthesis. The Plant Cell 16: 3098–3109. 1069 Adler E. 1977. Lignin chemistry – past, present and future. Wood Science and Technology 3: 169–218. Alejandro S, Lee Y, Tohge T, et al. 2012. AtABCG29 is a monolignol transporter involved in lignin biosynthesis. Current Biology 22: 1207–1212. Bao W, O’Malley DM, Whetten R, Sederoff RR. 1993. A laccase associated with lignification in loblolly pine xylem. Science 260: 672–674. Bassard JE, Richert L, Geerinck J, et al. 2012. Protein–protein and protein– membrane associations in the lignin pathway. The Plant Cell 24: 4465–4482. Bell-Lelong DA, Cusumano JC, Meyer K, Chapple C. 1997. Cinnamate-4hydroxylase expression in Arabidopsis (regulation in response to development and the environment). Plant Physiology 113: 729–738. Berthet S, Demont-Caulet N, Pollet B, et al. 2011. Disruption of LACCASE4 and 17 results in tissue-specific alterations to lignification of Arabidopsis thaliana stems. The Plant Cell 23: 1124–1137. Bewley JD. 1997. Breaking down the walls – a role for endo-b-mannanase in release from seed dormancy? Trends in Plant Science 2: 464–469. Blanco-Portales R, Medina-Escobar N, López-Ráez JA, et al. 2002. Cloning, expression and immunolocalization pattern of a cinnamyl alcohol dehydrogenase gene from strawberry (Fragaria ananassa cv. Chandler). Journal of Experimental Botany 53: 1723–1734. Blee KA, Choi JW, O’Connell AP, Schuch W, Lewis NG, Bolwell GP. 2003. A lignin-specific peroxidase in tobacco whose antisense suppression leads to vascular tissue modification. Phytochemistry 64: 163–176. Blodgett JT, Herms DA, Bonello P. 2005. Effects of fertilization on red pine defense chemistry and resistance to Sphaeropsis sapinea. Forest Ecology and Management 208: 373–382. Blokhina OB, Chirkova TV, Fagerstedt KV. 2001. Anoxic stress leads to hydrogen peroxide formation in plant cells. Journal of Experimental Botany 52: 1179–1190. Boerjan W, Ralph J, Baucher M. 2003. Lignin biosynthesis. Annual Review of Plant Biology, 54: 519–546. Boija E, Johansson G. 2006. Interactions between model membranes and lignin-related compounds studied by immobilized liposome chromatography. Biochimica et Biophysica Acta 1758: 620–626. Boija E, Lundquist A, Edwards K, Johansson G. 2007. Evaluation of bilayer disks as plant cell membrane models in partition studies. Analytical Biochemistry 364: 145–152. Bonawitz ND, Chapple C. 2010. The genetics of lignin biosynthesis: connecting genotype to phenotype. Annual Review of Genetics 44: 337–363 Bonawitz ND, Kim JI, Tobimatsu Y, et al. 2014. Disruption of Mediator rescues the stunted growth of a lignin-deficient Arabidopsis mutant. Nature 509: 376–380. Brown DM, Leo AHZ, Ellis J, Goodacre R, Turner SR. 2005. Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. The Plant Cell 17: 2281–2295. Brunow G, Lundquist K. 1980. Comparison of a synthetic dehydrogenation polymer of coniferyl alcohol with milled wood lignin from spruce, using 1H NMR spectroscopy. Paperi ja Puu 62: 669–672. Cabané M, Pireaux JC, Léger E, et al. 2004. Condensed lignins are synthesized in poplar leaves exposed to ozone. Plant Physiology 134: 586–594. Cahill DM, McComb JA. 1992. A comparison of changes in phenylalanine ammonia-lyase activity, lignin and phenolic synthesis in the roots of Eucalyptus calophylla (field resistant) and E. marginata (susceptible) when infected with Phytophthora cinnamomi. Physiological and Molecular Plant Pathology 40: 315–332. Cai C, Xu C, Li X, Ferguson I, Chen K. 2006. Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharvest Biology and Technology 40: 163–169. Caparrós-Ruiz D, Fornalé S, Civardi L, Puigdomènech P, Rigau J. 2006. Isolation and characterisation of a family of laccases in maize. Plant Science 171: 217–225. Capellades M, Torres MA, Bastisch I, et al. 1996. The maize caffeic acid O-methyltransferase gene promoter is active in transgenic tobacco and maize plant tissues. Plant Molecular Biology 31: 307–322. Chaffey N, Barlow P, Sundberg B. 2002. Understanding the role of the cytoskeleton in wood formation in angiosperm trees: hybrid aspen (Populus tremulaP. tremuloides) as the model species. Tree Physiology 22: 239–249. Chapelle A, Morreel K, Vanholme R, et al. 2012. Impact of the absence of stem-specific b-glucosidases on lignin and monolignols. Plant Physiology 160: 1204–1217. 1070 Barros et al. — The cell biology of lignification in higher plants Chen CH, Meyermans H, Burggraeve B, et al. 2000. Cell-specific and conditional expression of caffeoyl-coenzyme A-3-O-methyltransferase in poplar. Plant Physiology 123: 853–868. Chen F, Tobimatsu Y, Havkin-Frenkel D, Dixon RA, Ralph J. 2012. A polymer of caffeyl alcohol in plant seeds. Proceedings of the National Academy of Sciences, USA 109: 1772–1777. Chen F, Tobimatsu Y, Jackson L, Nakashima J, Ralph J, Dixon RA. 2013. Novel seed coat lignins in the Cactaceae: structure, distribution and implications for the evolution of lignin diversity. The Plant Journal 73: 201–211. Chen HC, Li Q, Shuford CM, et al. 2011. Membrane protein complexes catalyze both 4-and 3-hydroxylation of cinnamic acid derivatives in monolignol biosynthesis. Proceedings of the National Academy of Sciences, USA 108: 21253–21258. Coy MR, Salem TZ, Denton JS, et al. 2010. Phenol-oxidizing laccases from the termite gut. Insect Biochemistry and Molecular Biology 40: 723–732. Crestini C, Crucianelli M, Orlandi M, Saladino R. 2010. Oxidative strategies in lignin chemistry: a new environmental friendly approach for the functionalisation of lignin and lignocellulosic fibers. Catalysis Today 156: 8–22. D’Autréaux B, Toledano MB. 2007. ROS as signaling molecules: mechanisms that generate specificity in ROS homeostasis. Nature Reviews Molecular Cell Biology 8: 813–824. Davey MP, Bryant DN, Cummins I, et al. 2004. Effects of elevated CO2 on the vasculature and phenolic secondary metabolism of Plantago maritima. Phytochemistry 65: 2197–2204. Davin LB, Lewis NG. 1992. Phenylpropanoid metabolism: biosynthesis of monolignols, lignans and neolignans, lignins and suberins. In: HA Stafford, RK, Ibrahim, eds. Phenolic metabolism in plants. New York: Springer: 325–375. Davin LB, Lewis NG. 2000. Dirigent proteins and dirigent sites explain the mystery of specificity of radical precursor coupling in lignan and lignin biosynthesis. Plant Physiology 123: 453–462. Dean JF, Eriksson KEL. 1994. Laccase and the deposition of lignin in vascular plants. Holzforschung-International Journal of the Biology, Chemistry, Physics and Technology of Wood 48: 21–33. Dean JF, LaFayette PR, Rugh C, et al. 1998. Laccases associated with lignifying vascular tissues. ACS Symposium Series 697: 96–108. Debeaujon I, Lepiniec L, Pourcel L, Routaboul JM. 2007. Seed coat development and dormancy. Seed development, dormancy and germination. Annual Plant Reviews 27: 25–49. Delessert C, Wilson I, Van Der Straeten D, Dennis E, Dolferus R. 2004. Spatial and temporal analysis of the local response to wounding. Plant Molecular Biology 55: 165–181. Del Rı́o JC, Rencoret J, Prinsen P, Martı́nez ÁT, Ralph J, Gutiérrez A. 2012. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. Journal of Agricultural and Food Chemistry 60: 5922–5935. Demont-Caulet N, Lapierre C, Jouanin L, Baumberger S, Méchin V. 2010. Arabidopsis peroxidase-catalyzed copolymerization of coniferyl and sinapyl alcohols: kinetics of an endwise process. Phytochemistry 71: 1673–1683. De Pinto MC, Ros Barceló A. 1997. Cytochemical localization of phenoloxiding enzymes in lignifying Coleus blumei stems. European Journal of Histochemistry 41: 17–22. Do CT, Pollet B, Thévenin L, et al. 2007. Both caffeoyl Coenzyme A 3O-methyltransferase 1 and caffeic acid O-methyltransferase 1 are involved in redundant functions for lignin, flavonoids and sinapoyl malate biosynthesis in Arabidopsis. Planta 226: 1117–1129. Donaldson LA. 2001. Lignification and lignin topochemistry – an ultrastructural view. Phytochemistry 57: 859–873. Donaldson LA, Singh AP, Yoshinaga A, Takabe K. 1999. Lignin distribution in mild compression wood of Pinus radiata. Canadian Journal of Botany 77: 41–50. Dwivedi UN, Singh P, Pandey VP, Kumar A. 2011. Structure–function relationship among bacterial, fungal and plant laccases. Journal of Molecular Catalysis B: Enzymatic 68: 117–128. Ek M, Gellerstedt G, and Henriksson G. 2009. Lignin In: G Henriksson, ed. Pulp and paper chemistry and technology, Volume 1: Wood chemistry and biotechnology. Walter de Gruyter GmbH & Co. KG, 121–124. El Kayal W, Keller G, Debayles C, et al. 2006. Regulation of tocopherol biosynthesis through transcriptional control of tocopherol cyclase during cold hardening in Eucalyptus gunnii. Physiologia Plantarum 126: 212–223. Fagerstedt KV, Kukkola EM, Koistinen VV, Takahashi J, Marjamaa K. 2010. Cell wall lignin is polymerised by class III secretable plant peroxidases in Norway spruce. Journal of Integrative Plant Biology 52: 186–194. Fan L, Linker R, Gepstein S, Tanimoto E, Yamamoto R, Neumann PM. 2006. Progressive inhibition by water deficit of cell wall extensibility and growth along the elongation zone of maize roots is related to increased lignin metabolism and progressive stelar accumulation of wall phenolics. Plant Physiology 140: 603–612. Fernández-Pérez F, Pomar F, Pedreño MA, Novo-Uzal E. 2014. The suppression of AtPrx52 affects fibers but not xylem lignification in Arabidopsis by altering the proportion of syringyl units. Physiologia Plantarum (in press). Fernández-Pérez F, Vivar T, Pomar F, Pedreño MA, Novo-Uzal E. 2015. Peroxidase 4 is involved in syringyl lignin formation in Arabidopsis thaliana. Journal of Plant Physiology 175: 86–94. Franke R, McMichael CM, Meyer K, Shirley AM, Cusumano JC, Chapple C. 2000. Modified lignin in tobacco and poplar plants over-expressing the Arabidopsis gene encoding ferulate 5-hydroxylase. The Plant Journal 22: 223–234. Fromm J, Rockel B, Lautner S, Windeisen E, Wanner G. 2003. Lignin distribution in wood cell walls determined by TEM and backscattered SEM techniques. Journal of Structural Biology 143: 77–84. Fujita M, Harada H. 1979. Autoradiographic investigations of cell wall development. II. Tritiated phenylalanine and ferulic acid assimilation in relation to lignification. Mokuzai Gakkaishi 25: 89–94. Fukuda H. 1997. Tracheary element differentiation. The Plant Cell 9: 1147. Gansert D. 2003. Xylem sap flow as a major pathway for oxygen supply to the sapwood of birch (Betula pubescens Eh.). Plant, Cell and Environment 26: 1803–1814. Gavnholt B, Larsen K. 2002. Molecular biology of plant laccases in relation to lignin formation. Physiologia Plantarum 116: 273–280. Geldner N. 2013. The endodermis. Annual Review of Plant Biology 64: 531–558. Gibson LJ. 2012. The hierarchical structure and mechanics of plant materials. Journal of the Royal Society Interface 9: 2749–2766. Gierlinger N. 2014. Revealing changes in molecular composition of plant cell walls on the micron-level by Raman mapping and vertex component analysis (VCA). Frontiers in Plant Science 5: 306. Gill SS, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48: 909–930. Gorzsás A, Stenlund H, Persson P, Trygg J, Sundberg B. 2011. Cell-specific chemotyping and multivariate imaging by combined FT-IR microspectroscopy and orthogonal projections to latent structures (OPLS) analysis reveals the chemical landscape of secondary xylem. The Plant Journal 66: 903–914. Goujon T, Sibout R, Pollet B, et al. 2003. A new Arabidopsis thaliana mutant deficient in the expression of O-methyltransferase impacts lignins and sinapoyl esters. Plant Molecular Biology 51: 973–989. Grabber JH, Schatz PF, Kim H, Lu F, Ralph J. 2010. Identifying new lignin bioengineering targets: 1. Monolignol-substitute impacts on lignin formation and cell wall fermentability. BMC Plant Biology, 10: 114. Gray-Mitsumune M, Molitor EK, Cukovic D, Carlson JE, Douglas CJ. 1999. Developmentally regulated patterns of expression directed by poplar PAL promoters in transgenic tobacco and poplar. Plant Molecular Biology 39: 657–669. Guerra A, Ferraz A, Cotrim AR, da Silva FT. 2000. Polymerization of lignin fragments contained in a model effluent by polyphenoloxidases and horseradish peroxidase/hydrogen peroxide system. Enzyme and Microbial Technology 26: 315–323. Gui J, Shen J, Li L. 2011. Functional characterization of evolutionarily divergent 4-coumarate: coenzyme A ligases in rice. Plant Physiology 157: 574–586. Guo D, Chen F, Dixon RA. 2002. Monolignol biosynthesis in microsomal preparations from lignifying stems of alfalfa (Medicago sativa L.). Phytochemistry 61: 657–667. Halls SC, Lewis NG. 2002. Secondary and quaternary structures of the (þ)pinoresinol-forming dirigent protein. Biochemistry 41: 9455–9461. Hano C, Addi M, Bensaddek L, et al. 2006. Differential accumulation of monolignol-derived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta 223: 975–989. Harding SA, Leshkevich J, Chiang VL, Tsai CJ. 2002. Differential substrate inhibition couples kinetically distinct 4-coumarate:coenzyme A ligases with spatially distinct metabolic roles in quaking aspen. Plant Physiology 128: 428–438. Harkin JM. 1967. Lignin – a natural polymeric product of phenol oxidation. In: WI Taylor, AR Battersby, eds. Oxidative coupling of ohenols, New York: Marcel Dekker, 243–321. Barros et al. — The cell biology of lignification in higher plants Hausman JF, Evers D, Thiellement H, Jouve L. 2000. Compared responses of poplar cuttings and in vitro raised shoots to short-term chilling treatments. Plant Cell Reports 19: 954–960. Hawkins S, Boudet A. 2003. Defence lignin and hydroxycinnamyl alcohol dehydrogenase activities in wounded Eucalyptus gunnii. Forest Pathology 33: 91–104. Hepler PK, Fosket DE, Newcomb EH. 1970. Lignification during secondary wall formation in Coleus: an electron microscopic study. American Journal of Botany 57: 85–96. Herbette S, Bouchet B, Brunel N, Bonnin E, Cochard H, Guillon F. 2015. Immunolabelling of intervessel pits for polysaccharides and lignin helps in understanding their hydraulic properties in Populus tremula alba. Annals of Botrany 115: 187–199. Herrero J, Fernández-Pérez F, Yebra T, et al. 2013. Bioinformatic and functional characterization of the basic peroxidase 72 from Arabidopsis thaliana involved in lignin biosynthesis. Planta 237: 1599–1612. Higuchi T. 1990. Lignin biochemistry: biosynthesis and biodegradation. Wood Science and Technology 24: 23–63. Higuchi T, Ito Y. 1958. Dehydrogenation products of coniferyl alcohol formed by the action of mushroom phenol oxidase, rhus-laccase and radish peroxidase. Journal of Biochemistry 45: 575–579. Hilal M, Parrado MF, Rosa M, et al. 2004. Epidermal lignin deposition in quinoa cotyledons in response to UV-B radiation. Photochemistry and Photobiology 79: 205–210. Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H. 2001. A large family of class III plant peroxidases. Plant and Cell Physiology, 42: 462–468. Hoffmann L, Besseau S, Geoffroy P, et al. 2004. Silencing of hydroxycinnamoyl-coenzyme A shikimate/quinate hydroxycinnamoyl transferase affects phenylpropanoid biosynthesis. The Plant Cell 16: 1446–1465. Hosmani PS, Kamiya T, Danku J, et al. 2013. Dirigent domain-containing protein is part of the machinery required for formation of the lignin-based Casparian strip in the root. Proceedings of the National Academy of Sciences, USA 110: 14498–14503. Hosokawa M, Suzuki S, Umezawa T, Sato Y. 2001. Progress of lignification mediated by intercellular transportation of monolignols during tracheary element differentiation of isolated Zinnia mesophyll cells. Plant and Cell Physiology 42: 959–968. Hsieh LS, Ma GJ, Yang CC, Lee PD. 2010. Cloning, expression, site-directed mutagenesis and immunolocalization of phenylalanine ammonia-lyase in Bambusa oldhamii. Phytochemistry 71: 1999–2009. Hu WJ, Kawaoka A, Tsai CJ, et al. 1998. Compartmentalized expression of two structurally and functionally distinct 4-coumarate:CoA ligase genes in aspen (Populus tremuloides). Proceedings of the National Academy of Sciences, USA 95: 5407–5412. Ito Y, Tokunaga N, Sato Y, Fukuda H. 2004. Transfer of phenylpropanoids via the medium between xylem cells in Zinnia xylogenic culture. Plant Biotechnoogy 21: 205–213. Jones L, Ennos AR, Turner SR. 2001. Cloning and characterization of irregular xylem4 (IRX4): a severely lignin-deficient mutant of Arabidopsis. The Plant Journal 26: 205–216. Kaneda M, Rensing KH, Wong JC, Banno B, Mansfield SD, Samuels AL. 2008. Tracking monolignols during wood development in lodgepole pine. Plant Physiology 147: 1750–1760. Kärkönen A, Koutaniemi S, Mustonen M, et al. 2002. Lignification related enzymes in Picea abies suspension cultures. Physiologia Plantarum 114: 343–353. Karlsson M, Melzer M, Prokhorenko I, Johansson T, Wingsle G. 2005. Hydrogen peroxide and expression of hipI-superoxide dismutase are associated with the development of secondary cell walls in Zinnia elegans. Journal of Experimental Botany 56: 2085–2093. Karpinska B, Karlsson M, Schinkel H, et al. 2001. A novel superoxide dismutase with a high isoelectric point in higher plants. Expression, regulation, and protein localization. Plant Physiology 126: 1668–1677. Kawasaki T, Henmi K, Ono E, et al. 1999. The small GTP-binding protein Rac is a regulator of cell death in plants. Proceedings of the National Academy of Sciences, USA 96: 10922–10926. Kawasaki T, Koita H, Nakatsubo T, et al. 2006. Cinnamoyl-CoA reductase, a key enzyme in lignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proceedings of the National Academy of Sciences, USA 103: 230–235. Kelly KM, Van Staden J, Bell WE. 1992. Seed coat structure and dormancy. Plant Growth Regulation 11: 201–209. 1071 Kersey R, Inoue K, Schubert KR, Dixon RA. 1999. Immunolocalization of two lignin O-methyltransferases in stems of alfalfa (Medicago sativa L.). Protoplasma 209: 46–57. Kim YJ, Kim DG, Lee SH, Lee I. 2006. Wound-induced expression of the ferulate 5-hydroxylase gene in Camptotheca acuminata. Biochimica et Biophysica Acta 1760: 182–190. Kotula L, Ranathunge K, Schreiber L, Steudle E. 2009. Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. Journal of Experimental Botany 60: 2155–2167. Koutaniemi S, Malmberg HA, Simola LK, Teeri TH, Kärkönen A. 2015. Norway spruce (Picea abies) laccases; characterisation of a laccase in a lignin-forming tissue culture. Journal of Integrative Plant Biology (in press). Kwon M, Burlat V, Davin LB, Lewis NG. 1999. Localization of dirigent protein involved in lignan biosynthesis: implications for lignification at the tissue and subcellular level. In: GG Gross, RW Hemingway, T Yoshida, eds. Plant polyphenols 2: chemistry, biology, pharmacology, ecology. New York: Kluwer Academic Publishers/Plenum Publishers, 393–411. Lacombe E, Hawkins S, Doorsselaere J, et al. 1997. Cinnamoyl CoA reductase, the first committed enzyme of the lignin branch biosynthetic pathway: cloning, expression and phylogenetic relationships. The Plant Journal 11: 429–441. Lagrimini LM, Burkhart W, Moyer M, Rothstein S. 1987. Molecular cloning of complementary DNA encoding the lignin-forming peroxidase from tobacco: molecular analysis and tissue-specific expression. Proceedings of the National Academy of Sciences, USA 84: 7542–7546. Lamport DTA. 1986. Roles for peroxidases in cell wall genesis. In: H Greppin, C Penel, T Gaspar, eds. Molecular and physiological aspects of plant peroxidases. Geneva, Switzerland: Université de Genève, 199–208. Lan W, Lu F, Regner M, et al. 2015. Tricin, a flavonoid monomer in monocot lignification. Plant Physiology (in press). Lange BM, Lapierre C, Sandermann H. 1995. Elicitor-induced spruce stress lignin (structural similarity to early developmental lignins). Plant Physiology 108: 1277–1287. Lanot A, Hodge D, Jackson RG, et al. 2006. The glucosyltransferase UGT72E2 is responsible for monolignol 4-O-glucoside production in Arabidopsis thaliana. The Plant Journal 48: 286–295. Lauvergeat V, Lacomme C, Lacombe E, Lasserre E, Roby D, GrimaPettenati J. 2001. Two cinnamoyl-CoA reductase (CCR) genes from Arabidopsis thaliana are differentially expressed during development and in response to infection with pathogenic bacteria. Phytochemistry 57: 1187–1195. Lee D, Ellard M, Wanner LA, Davis KR, Douglas CJ. 1995. The Arabidopsis thaliana 4-coumarate: CoA ligase (4CL) gene: stress and developmentally regulated expression and nucleotide sequence of its cDNA. Plant Molecular Biology 28: 871–884. Lee Y, Rubio MC, Alassimone J, Geldner N. 2013. A mechanism for localized lignin deposition in the endodermis. Cell 153: 402–412. Li L, Osakabe Y, Joshi CP, Chiang VL. 1999. Secondary xylem-specific expression of caffeoyl-coenzyme A 3-O-methyltransferase plays an important role in the methylation pathway associated with lignin biosynthesis in loblolly pine. Plant Molecular Biology 40: 555–565. Li L, Cheng XF, Leshkevich J, Umezawa T, Harding SA, Chiang VL. 2001. The last step of syringyl monolignol biosynthesis in angiosperms is regulated by a novel gene encoding sinapyl alcohol dehydrogenase. The Plant Cell 13: 1567–1586. Li Y, Kajita S, Kawai S, Katayama Y, Morohoshi N. 2003. Down-regulation of an anionic peroxidase in transgenic aspen and its effect on lignin characteristics. Journal of Plant Research 116: 175–182. Liang M, Davis E, Gardner D, Cai X, Wu Y. 2006. Involvement of AtLAC15 in lignin synthesis in seeds and in root elongation of Arabidopsis. Planta 224: 1185–1196. Liochev SI, Fridovich I. 1994. The role of O2 in the production of HO: in vitro and in vivo. Free Radical Biology and Medicine 16: 29–33 Liu L, Dean JF, Friedman WE, Eriksson KEL. 1994. A laccase-like phenoloxidase is correlated with lignin biosynthesis in Zinnia elegans stem tissues. The Plant Journal 6: 213–224. Liu CJ. 2012. Deciphering the enigma of lignification: precursor transport, oxidation, and the topochemistry of lignin assembly. Molecular Plant 5: 304–317. Lu F, Marita JM, Lapierre C, et al. 2010. Sequencing around 5-hydroxyconiferyl alcohol-derived units in caffeic acid O-methyltransferase-deficient poplar lignins. Plant Physiology 153: 569–79. 1072 Barros et al. — The cell biology of lignification in higher plants Lu S, Li Q, Wei H, et al. 2013. Ptr-miR397a is a negative regulator of laccase genes affecting lignin content in Populus trichocarpa. Proceedings of the National Academy of Sciences, USA 110: 10848–10853. Lucas WJ, Groover A, Lichtenberger R, et al. 2013. The plant vascular system: evolution, development and functions. Journal of Integrative Plant Biology 55: 294–388. Mäder M, Nessel A, Boff M. 1977. On the physiological significance of the isozyme groups of peroxidase from tobacco demonstrated by biochemical properties. Zeitschrift für Pflanzenphysiology 82: 247–260. Mäder M, Ungemach J, Schloß P. 1980. The role of peroxidase isoenzyme groups of Nicotiana tabacum in hydrogen peroxide formation. Planta 147: 467–470. Makkar RS, Tsuneda A, Tokuyasu K, Mori Y. 2001. Lentinula edodes produces a multicomponent protein complex containing manganese (II)dependent peroxidase, laccase and b-glucosidase. FEMS Microbiology Letters 200: 175–179. Mansfield SD, Kim H, Lu F, Ralph J. 2012. Whole plant cell wall characterization using solution-state 2D NMR. Nature Protocols 7: 1579–1589. Marita JM, Ralph J, Hatfield RD, Guo D, Chen F, Dixon RA. 2003. Structural and compositional modifications in lignin of transgenic alfalfa down-regulated in caffeic acid 3-O-methyltransferase and caffeoyl coenzyme A 3-O-methyltransferase. Phytochemistry 62: 53–65. Martı́n JA, Solla A, Woodward S, Gil L. 2007. Detection of differential changes in lignin composition of elm xylem tissues inoculated with Ophiostoma novo-ulmi using Fourier transform-infrared spectroscopy. Forest Pathology 37: 187–191. Ménard D, Pesquet E. 2015. Cellular interactions during tracheary elements formation and function. Current Opinion in Plant Biology 23: 109–115. Miao YC, Liu CJ. 2010. ATP-binding cassette-like transporters are involved in the transport of lignin precursors across plasma and vacuolar membranes. Proceedings of the National Academy of Sciences, USA 107: 22728–22733. Min D, Chang H, Jameel H, Lucia L, Wang Z, Jin Y. 2014. The structure of lignin of corn stover and its changes induced by mild sodium hydroxide treatment. BioResources 9: 2405–2414. Mir Derikvand M, Berrio Sierra J, Ruel K, et al. 2008. Redirection of the phenylpropanoid pathway to feruloyl malate in Arabidopsis mutants deficient for cinnamoyl-CoA reductase 1. Planta 227: 943–956. Moerschbacher BM, Noll U, Gorrichon L, Reisener HJ. 1990. Specific inhibition of lignification breaks hypersensitive resistance of wheat to stem rust. Plant Physiology 93: 465–470. Moinuddin SG, Jourdes M, Laskar DD, et al. 2010. Insights into lignin primary structure and deconstruction from Arabidopsis thaliana COMT (caffeic acid O-methyl transferase) mutant Atomt1. Organic and Biomolecular Chemistry 8: 3928–3946. Morreel K, Ralph J, Kim H, et al. 2004. Profiling of oligolignols reveals monolignol coupling conditions in lignifying poplar xylem. Plant Physiology 136: 3537–3549. Moura JC, Silva M, Bonine CSV, Viana JOF, Dornelas MC, Mazzafera P. 2010. Abiotic and biotic stresses and changes in the lignin content and composition in plants. Journal of Integrative Plant Biology 52: 360–376. Nair RB, Xia Q, Kartha CJ, et al. 2002. Arabidopsis CYP98A3 mediating aromatic 3-hydroxylation. Developmental regulation of the gene, and expression in yeast. Plant Physiology 130: 210–220. Nakanomyo I, Kost B, Chua NH, Fukuda H. 2002. Preferential and asymmetrical accumulation of a Rac small GTPase mRNA in differentiating xylem cells of Zinnia elegans. Plant and Cell Physiology 43: 1484–1492. Nakashima J, Awano T, Takabe K, Fujita M, Saiki H. 1997. Immunocytochemical localization of phenylalanine ammonia-lyase and cinnamyl alcohol dehydrogenase in differentiating tracheary elements derived from zinnia mesophyll cells. Plant and Cell Physiology 38: 113–123. Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, Geldner N. 2012. Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proceedings of the National Academy of Sciences, USA 109: 10101–10106. Oda Y, Iida Y, Kondo Y, Fukuda H. 2010. Wood cell-wall structure requires local 2D-microtubule disassembly by a novel plasma membrane-anchored protein. Current Biology 20: 1197–1202. Ogawa KI, Kanematsu S, Asada K. 1997. Generation of superoxide anion and localization of CuZn-superoxide dismutase in the vascular tissue of spinach hypocotyls: their association with lignification. Plant and Cell Physiology 38: 1118–1126. Ohl S, Hedrick SA, Chory J, Lamb CJ. 1990. Functional properties of a phenylalanine ammonia-lyase promoter from Arabidopsis. The Plant Cell 2: 837–848. Onnerud H, Zhang L, Gellerstedt G, Henriksson G. 2002. Polymerization of monolignols by redox shuttle-mediated enzymatic oxidation: a new model in lignin biosynthesis I. The Plant Cell 14: 1953–1962. Osakabe Y, Nanto K, Kitamura H, et al . 2006. Immunological detection and cellular localization of the phenylalanine ammonia-lyase of a hybrid aspen. Plant Biotechnology 23: 399–404. Østergaard L, Teilum K, Mirza O, et al. 2000. Arabidopsis ATP A2 peroxidase. Expression and high-resolution structure of a plant peroxidase with implications for lignification. Plant Molecular Biology 44: 231–243. Overmyer K, Tuominen H, Kettunen R, et al. 2000. Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. The Plant Cell 12: 1849–1862. Passardi F, Penel C, Dunand C. 2004. Performing the paradoxical: how plant peroxidases modify the cell wall. Trends in Plant Science 9: 534–540. Pedreira J, Herrera MT, Zarra I, Revilla G. 2010. The overexpression of AtPrx37, an apoplastic peroxidase, reduces growth in Arabidopsis. Physiologia Plantarum 141: 177–87. Pesquet E, Lloyd C. 2011. Microtubules, MAPs and xylem formation. In: B Liu, ed. The plant cytoskeleton. New York: Springer, 277–306. Pesquet E, Korolev AV, Calder G, Lloyd CW. 2010. The microtubuleassociated protein AtMAP70-5 regulates secondary wall patterning in Arabidopsis wood cells. Current Biology 20: 744–749. Pesquet E, Zhang B, Gorzsás A, et al. 2013. Non-cell-autonomous postmortem lignification of tracheary elements in Zinnia elegans. The Plant Cell 25: 1314–1328. Petrik DL, Karlen SD, Cass CL, et al. 2013. p-Coumaroyl-CoA:monolignol transferase (PMT) acts specifically in the lignin biosynthetic pathway in Brachypodium distachyon. The Plant Journal 77: 713–726. Pickett-Heaps JD,Vanholme R, Cesarino I, et al. 1968. Xylem wall deposition. Protoplasma 65: 181–205. Quentin M, Allasia V, Pegard A, et al. 2009. Imbalanced lignin biosynthesis promotes the sexual reproduction of homothallic oomycete pathogens. PLoS Pathogens 5: e1000264. Ralph J, Brunow G, Harris PJ, Dixon RA, Schatz PF, Boerjan W. 2008. Lignification: are lignins biosynthesized via simple combinatorial chemistry or via proteinaceous control and template replication? Recent Advances in Polyphenol Research 1: 36–66. Ranocha P, McDougall G, Hawkins S, et al. 1999. Biochemical characterization, molecular cloning and expression of laccases – a divergent gene family – in poplar. European Journal of Biochemistry 259: 485–495. Ranocha P, Chabannes M, Chamayou S, et al. 2002. Laccase down-regulation causes alterations in phenolic metabolism and cell wall structure in poplar. Plant Physiology 129: 145–155. Rastogi S, Kumar R, Chanotiya CS, et al. 2013. 4-Coumarate:CoA ligase partitions metabolites for eugenol biosynthesis. Plant and Cell Physiology 54: 1238–1252. Richardson A, McDougall GJ. 1997. A laccase-type polyphenol oxidase from lignifying xylem of tobacco. Phytochemistry 44: 229–235. Ro DK, Mah N, Ellis BE, Douglas CJ. 2001. Functional characterization and subcellular localization of poplar (Populus trichocarpa Populus deltoides) cinnamate 4-hydroxylase. Plant Physiology 126: 317–329. Rogers LA, Dubos C, Cullis IF, et al. 2005. Light, the circadian clock, and sugar perception in the control of lignin biosynthesis. Journal of Experimental Botany 56: 1651–1663. Roppolo D, De Rybel B, Tendon VD, et al. 2011. A novel protein family mediates Casparian strip formation in the endodermis. Nature 473: 380–383. Ros Barceló A. 1998. The generation of H2O2 in the xylem of Zinnia elegans is mediated by an NADPH-oxidase-like enzyme. Planta 207: 207–216. Ros Barceló A. 2005. Xylem parenchyma cells deliver the H2O2 necessary for lignification in differentiating xylem vessels. Planta 220: 747–756. Rozema J, van de Staaij J, Björn LO, Caldwell M. 1997. UV-B as an environmental factor in plant life: stress and regulation. Trends in Ecology and Evolution 12: 22–28. Ruelland E, Campalans A, Selman-Housein G, Puigdomènech P, Rigau J. 2003. Cellular and subcellular localization of the lignin biosynthetic enzymes caffeic acid O-methyltransferase, cinnamyl alcohol dehydrogenase and cinnamoyl-Coenzyme A reductase in two monocots, sugarcane and maize. Physiologia Plantarum 117: 93–99. Barros et al. — The cell biology of lignification in higher plants Sagi M, Fluhr R. 2006. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiology 141: 336–340. Š amaj J, Hawkins S, Lauvergeat V, Grima-Pettenati J, Boudet A. 1998. Immunolocalization of cinnamyl alcohol dehydrogenase 2 (CAD 2) indicates a good correlation with cell-specific activity of CAD 2 promoter in transgenic poplar shoots. Planta 204: 437–443. Santiago LJM, Louro RP, De Oliveira DE. 2000. Compartmentation of phenolic compounds and phenylalanine ammonia-lyase in leaves of Phyllanthus tenellus Roxb. and their induction by copper sulphate. Annals of Botany 86: 1023–1032. Sato K, Nishikubo N, Mashino Y, et al. 2009. Immunohistochemical localization of enzymes that catalyze the long sequential pathways of lignin biosynthesis during differentiation of secondary xylem tissues of hybrid aspen (Populus sieboldii Populus grandidentata). Tree Physiology 29: 1599–1606. Sato T, Takabe K, Fujita M. 2004. Immunolocalization of phenylalanine ammonia-lyase and cinnamate-4-hydroxylase in differentiating xylem of poplar. Comptes Rendus Biologies 327: 827–836. Sato Y, Demura T, Yamawaki K, et al. 2006. Isolation and characterization of a novel peroxidase gene ZPO-C whose expression and function are closely associated with lignification during tracheary element differentiation. Plant and Cell Physiology 47: 493–503. Schöpker H, Kneisel M, Beerhues L, Robenek H, Wiermann R. 1995. Phenylalanine ammonia-lyase and chalcone synthase in glands of Primula kewensis (W. Wats): immunofluorescence and immunogold localization. Planta 196: 712–719. Schuetz M, Benske A, Smith RA, et al. 2014. Laccases direct lignification in the discrete secondary cell wall domains of protoxylem. Plant Physiology 166: 798–807. Serk H, Gorzsás A,Tuominen H, Pesquet E. 2015. Cooperative lignification of xylem tracheary elements. Plant Signaling and Behavior (in press). Shi R, Sun YH, Li Q, Heber S, Sederoff R, Chiang VL. 2010. Towards a systems approach for lignin biosynthesis in Populus trichocarpa: transcript abundance and specificity of the monolignol biosynthetic genes. Plant and Cell Physiology 51: 144–163. Shigeto J, Kiyonaga Y, Fujita K, Kondo R, Tsutsumi Y. 2013. Putative cationic cell-wall-bound peroxidase homologues in Arabidopsis, AtPrx2, AtPrx25, and AtPrx71, are involved in lignification. Journal of Agricultural and Food Chemistry 61: 3781–3788. Shigeto J, Itoh Y, Hirao S, Ohira K, Fujita K, Tsutsumi Y. 2015. Simultaneously disrupting AtPrx2, AtPrx25 and AtPrx71 alters lignin content and structure in Arabidopsis stem. Journal of Integrative Plant Biology (in press). Sibout R, Eudes A, Pollet B, et al. 2003. Expression pattern of two paralogs encoding cinnamyl alcohol dehydrogenases in Arabidopsis. Isolation and characterization of the corresponding mutants. Plant Physiology 132: 848–860. Smart CC, Amrhein N. 1985. The influence of lignification on the development of vascular tissue in Vigna radiata L. Protoplasma 124: 87–95. Smith CG, Rodgers MW, Zimmerlin A, Ferdinando D, Bolwell GP. 1994. Tissue and subcellular immunolocalisation of enzymes of lignin synthesis in differentiating and wounded hypocotyl tissue of French bean (Phaseolus vulgaris L.). Planta 192: 155–164. Smith RA, Schuetz M, Roach M, Mansfield SD, Ellis B, Samuels L. 2013. Neighboring parenchyma cells contribute to Arabidopsis xylem lignification, while lignification of interfascicular fibers is cell autonomous. The Plant Cell 25: 3988–3999. Srivastava V, Schinkel H, Witzell J, et al. 2007. Downregulation of lighisoelecric-point extracellular superoxide dismutase mediates alterations in the metabolism of reactive oxygen species and developmental disturbances in hybrid aspen. The Plant Journal 49: 135–148. Stange RR, Ralph J, Peng JP, Sims JJ, Midland SL, McDonald RE. 2001. Acidolysis and hot water extraction provide new insights into the composition of the induced ‘lignin-like’ material from squash fruit. Phytochemistry 57: 1005–1011. Sterjiades R, Dean JFD, Eriksson KEL. 1992. Laccase from sycamore maple (Acer pseudoplatanus) polymerizes monolignols. Plant Physiology 99: 1162–1168. Sterjiades R, Dean JFD, Gamble G, Himmelsbach DS, Eriksson KEL. 1993. Extracellular laccases and peroxidases from sycamore maple (Acer pseudoplatanus) cell-suspension cultures. Planta 190: 75–87. Steudle E. 2000. Water uptake by roots: effects of water deficit. Journal of Experimental Botany 51: 1531–1542. 1073 Sundin L, Vanholme R, Geerinck J, et al. 2014. Mutation of the inducible ARABIDOPSIS THALIANA CYTOCHROME P450 REDUCTASE2 alters lignin composition and improves saccharification. Plant Physiology 166: 1956–71. Tahara K, Norisada M, Hogetsu T, Kojima K. 2005. Aluminum tolerance and aluminum-induced deposition of callose and lignin in the root tips of Melaleuca and Eucalyptus species. Journal of Forest Research 10: 325–333. Takabe K, Fujita M, Harada H, Saiki H. 1985. Autoradiographic investigation of lignification in the cell walls of cryptomeria (Cryptomeria japonica D. Don). Mokuzai Gakkaishi 31: 613–619. Takabe K, Takeuchi M, Sato T, Ito M, Fujita M. 2001. Immunocytochemical localization of enzymes involved in lignification of the cell wall. Journal of Plant Research 114: 509–515. Takeuchi M, Takabe K, Fujita M. 2001. Immunolocalization of O-methyltransferase and peroxidase in differentiating xylem of poplar. Holzforschung 55: 146–150. Tanahashi M, Higuchi T. 1981. Dehydrogenative polymerization of monolignols by peroxidase and H2O2 in a dialysis tube. I. Preparation of highly polymerized DHPs. Wood Research: Bulletin of the Wood Research Institute Kyoto University 67: 29–42. Tao S, Khanizadeh S, Zhang H, Zhang S. 2009. Anatomy, ultrastructure and lignin distribution of stone cells in two Pyrus species. Plant Science 176: 413–419. Taylor JG, Owen TP, Koonce LT, Haigler CH. 1992. Dispersed lignin in tracheary elements treated with cellulose synthesis inhibitors provides evidence that molecules of the secondary cell wall mediate wall patterning. The Plant Journal 2: 959–970. Temp U, Eggert C. 1999. Novel interaction between laccase and cellobiose dehydrogenase during pigment synthesis in the white rot fungus Pycnoporus cinnabarinus. Applied Environmental Microbiology 65: 389–395. Terashima N, Fukushima K. 1988. Heterogeneity in formation of lignin. XI. An autographic study of heterogeneous formation and structure of pine lignin. Wood Science and Technology 22: 259–270. Terashima N, Fukushima K. 1989. Biogenesis and structure of macromolecular lignin in the cell wall of tree xylem as studied by microautoradiography. Plant Cell Wall Polymers: 160–168. Terashima N, Yoshida M, Hafrén J, Fukushima K, Westermark U. 2012. Proposed supramolecular structure of lignin in softwood tracheid compound middle lamella regions. Holzforschung 66: 907–915. Thévenin J, Pollet B, Letarnec B, et al. 2011. The simultaneous repression of CCR and CAD, two enzymes in the lignin biosynthetic pathway, results in sterility and dwarfism in Arabidopsis thaliana. Molecular Plant 4: 70–82. Tobimatsu Y, Chen F, Nakashima J, et al. 2013. Coexistence but independent biosynthesis of catechyl and guaiacyl/syringyl lignin polymers in seed coats. The Plant Cell 25: 2587–2600. Tokunaga N, Sakakibara N, Umezawa T, Ito Y, Fukuda H, Sato Y. 2005. Involvement of extracellular dilignols in lignification during tracheary element differentiation of isolated Zinnia mesophyll cells. Plant and Cell Physiology 46: 224–232. Tokunaga N, Kaneta T, Sato S, Sato Y. 2009. Analysis of expression profiles of three peroxidase genes associated with lignification in Arabidopsis thaliana. Physiologia Plantarum 136: 237–49. Torres MA, Jones JD, Dangl JL. 2006. Reactive oxygen species signaling in response to pathogens. Plant Physiology 141: 373–378. Tsuji Y, Fukushima K. 2004. Behavior of monolignol glucosides in angiosperms. Journal of Agricultural and Food Chemistry 52: 7651–7659. Tsutsumi Y, Matsui K, Sakai K. 1998. Substrate specific peroxidases in woody angiosperms and gymnosperms participate in regulating the dehydrogenative polymerisation of syringyl and guaiacyl type lignins. Holzforschung 52: 275–281. Tsuyama T, Kawai R, Shitan N, et al. 2013. Proton-dependent coniferin transport, a common major transport event in differentiating xylem tissue of woody plants. Plant Physiology 162: 918–926 Turlapati, PV, Kim KW, Davin LB, Lewis NG. 2011. The laccase multigene family in Arabidopsis thaliana: towards addressing the mystery of their gene function(s). Planta 233: 439–470. Turner SR, Somerville CR. 1997. Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. The Plant Cell 9: 689–701. Tyree MT, Zimmermann MH. 2002. Xylem structure and the ascent of sap. Berlin: Springer. 1074 Barros et al. — The cell biology of lignification in higher plants Valério L, De Meyer M, Penel C, Dunand C. 2004. Expression analysis of the Arabidopsis peroxidase multigenic family. Phytochemistry 65: 1331–1342. Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W. 2010. Lignin biosynthesis and structure. Plant Physiology 153: 895–905. Vanholme R, Cesarino I, Rataj K, et al. 2013. Caffeoyl shikimate esterase (CSE) is an enzyme in the lignin biosynthetic pathway in Arabidopsis. Science 341: 1103–1106. Vogt T. 2010. Phenylpropanoid biosynthesis. Molecular Plant 3: 2–20. Wang JP, Shuford CM, Li Q, et al. 2012. Functional redundancy of the two 5-hydroxylases in monolignol biosynthesis of Populus trichocarpa. LCMS/MS based protein quantification and metabolic flux analysis. Planta 236: 795–808. Watts HD, Mohamed MNA, Kubicki JD. 2011. Comparison of multistandard and TMS-standard calculated NMR shifts for coniferyl alcohol and application of the multistandard method to lignin dimers. Journal of Physical Chemistry B 115: 1958–1970 Weng JK, Chapple C. 2010. The origin and evolution of lignin biosynthesis. New Phytologist 187: 273–285. Whetten R, Sederoff R. 1995. Lignin biosynthesis. The Plant Cell 7: 1001–1013. White PJ, Broadley MR. 2001. Chloride in soils and its uptake and movement within the plant: a review. Annals of Botany 88: 967–988. Wilkerson CG, Mansfield SD, Lu F, et al. 2014. Monolignol ferulate transferase introduces chemically labile linkages into the lignin backbone. Science 344: 90–93. Wuyts N, Lognay R, Verscheure M, Marlier M, Waele DD, Swennen R. 2007. Potential physical and chemical barriers to infection by the burrowing nematode Radopholus similis in roots of susceptible and resistant banana (Musa spp.). Plant Pathology 56: 878–890. Yang F, Mitra P, Zhang L, et al. 2013. Engineering secondary cell wall deposition in plants. Plant Biotechnology Journal 11: 325–335. Ye ZH. 1997. Association of caffeoyl coenzyme A 3-O-methyltransferase expression with lignifying tissues in several dicot plants. Plant Physiology 115: 1341–1350. Yoshimura K, Masuda A, Kuwano M, Yokota A, Akashi K. 2008. Programmed proteome response for drought avoidance/tolerance in the root of a C3 xerophyte (wild watermelon) under water deficits. Plant and Cell Physiology 49: 226–241. Zeier J, Schreiber L. 1997. Chemical composition of hypodermal and endodermal cell walls and xylem vessels isolated from Clivia miniata (Identification of the biopolymers lignin and suberin). Plant Physiology 113: 1223–1231. Zeier J, Ruel K, Ryser U, Schreiber L. 1999. Chemical analysis and immunolocalisation of lignin and suberin in endodermal and hypodermal/ rhizodermal cell walls of developing maize (Zea mays L.) primary roots. Planta 209: 1–12. Zhao Q, Nakashima J, Chen F, et al. 2013. LACCASE is necessary and nonredundant with PEROXIDASE for lignin polymerization during vascular development in arabidopsis. The Plant Cell 25: 3976–3987. Zhong R, Morrison WH, Himmelsbach DS, Poole FL, Ye ZH. 2000. Essential role of caffeoyl coenzyme A O-methyltransferase in lignin biosynthesis in woody poplar plants. Plant Physiology 124: 563–578. Zhu Q, Dabi T, Beeche A, Yamamoto R, Lawton MA, Lamb C. 1995. Cloning and properties of a rice gene encoding phenylalanine ammonialyase. Plant Molecular Biology 29: 535–550. Zhu Y, Du B, Qian J, Zou B, Hua J. 2013. Disease resistance gene-induced growth inhibition is enhanced by rcd1 independent of defense activation in Arabidopsis. Plant Physiology 161: 2005–2013.